Introduction
There are a large number of things around us that we see and feel. For example, you can see the book in front of you. The book occupies some space. Space occupied by the book is called its volume. If you pick up the book, you can also feel its mass. You cannot see the air around you, yet if you feel a balloon with air and weigh it carefully,you will find that not only does air occupy space, but it also has mass. Things like a book, air, water, wood, cloth, etc., are examples of matter. Anything that occupies space and has mass, is called matter.
Substance
A substance is a kind of matter that cannot be separated into other kinds of matter by any physical process. For example, sugar dissolve in water can be separated from water by simply evaporating the water. Here sugar is a substance that cannot be broken into its components by any physical process. Similarly, sodium chloride, lime (calcium oxide), etc., are all substances.
Physical nature of matter.
- The matter is made of particles
- The particles of matter are too small
1. The matter is made of particles
The matter is made of discrete particles. This can be easily verified by performing the following activity.
Add some sugar to the water and dissolve it with the help of a glass rod. You will see that the sugar has disappeared, but there is no change in the level of water. The particles of sugar dissolved in water occupy the space between the particles of water. That is why the water level in the beaker did not rise. Had sugar been continuous like a block of wood, the water level in the beaker would have risen.
 |
| Solid dissolves in water, the volume of the water does not change. |
Add some sugar to the water and dissolve it with the help of a glass rod. You will see that the sugar has disappeared, but there is no change in the level of water. The particles of sugar dissolved in water occupy the space between the particles of water. That is why the water level in the beaker did not rise. Had sugar been continuous like a block of wood, the water level in the beaker would have risen.
2. The particles of matter are too small
Experiments
Take 2-3 crystals of potassium permanganate and dissolve them in 100 ml of water. Take out approximately 10 ml of this solution and put it into 90 ml of clear water. Take out 10 ml of this solution and put it into another 90 ml of clear water. Keep diluting the solution like this 5 to 8 times. Is the water still coloured ?
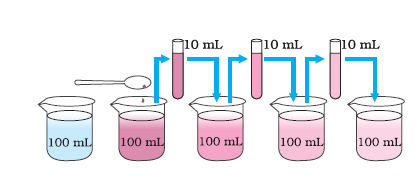 |
| With every dilution, though the colour becomes light, it is still visible |
This experiment shows that just a few crystals of potassium permanganate can colour a large volume of water (about 1000 L). So we conclude that there must be millions of tiny particles in just one crystal of potassium permanganate, which keeps on dividing itself into smaller and smaller particles.
The same activity can be done using 2 mL of Dettol instead of potassium permanganate. The smell can be detected even on repeated dilution
Characteristics of Particles of Matter
- Particles of matter have space between them.
- Particles of matter are continuously moving.
- Particles of matter attract each other.
1) Particles of matter have space between them
Particles of sugar, salt, Dettol, or potassium permanganate got evenly distributed in the water. Similarly, when we make tea, coffee or lemonade (nimbu paani ), particles of one type of matter get into the spaces between particles of the other. This shows that there is enough space between particles of matter.
Activity 1
➠ Put an unlit incense stick in a corner of your class. How close do you have to go near it so as to get its smell?
➠ Light the incense stick. What happens? Do you get the smell sitting at a distance?
Activity 2
➠ Take two glasses/beakers filled with water.
➠ Put a drop of blue or red ink slowly and carefully along the sides of the first beaker and honey in the same way in the second beaker.
➠ What do you observe immediately after adding the ink drop?
➠ What do you observe immediately after adding a drop of honey?
Activity 3
➠ Drop a crystal of copper sulphate or potassium permanganate into a glass of hot water and another containing cold water. Do not stir the solution.
➠ What do you observe just above the solid crystal in the glass?
➠ Does the rate of mixing change with temperature? Why and how?
2. Particles of matter are continuously moving
Particles of matter are continuously moving, that is, they possess what we call kinetic energy. As the temperature rises, particles move faster. So, we can say that with the increase in temperature, the kinetic energy of the particles also increases.
Particles of matter intermix on their own with each other. They do so by getting into the spaces between the particles.
Diffusion👉 The intermixing of particles of two different types of matter on their own is called diffusion.
We also observe that on heating, diffusion becomes faster.
States of Matter
👉Matter around us exists in three different
states– solid, liquid and gas.
👉These states of
matter arise due to the variation in the
characteristics of the particles of matter
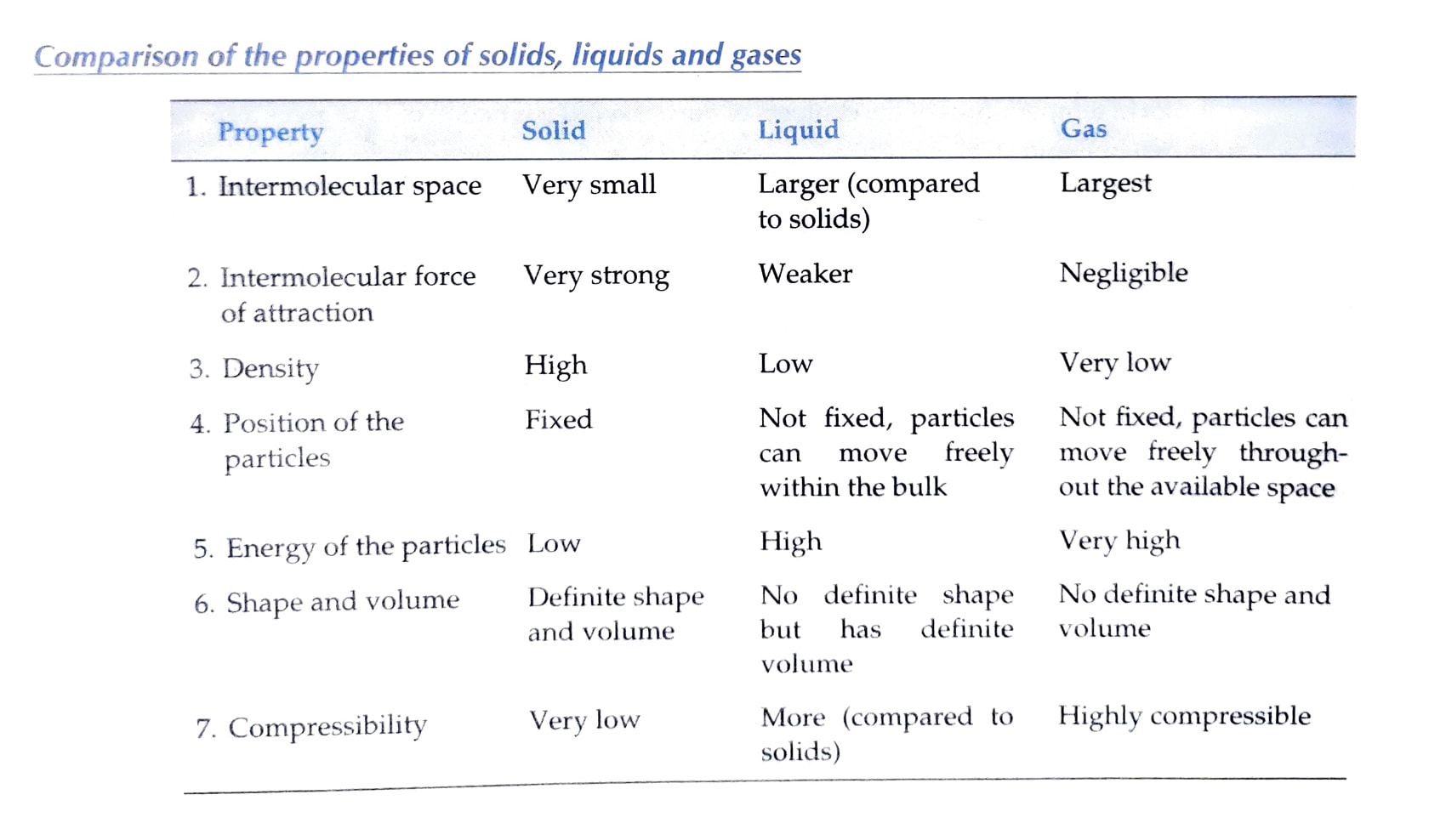
Properties of these three states of matter
Change of State of Matter
1. By changing the temperature
2. By changing the pressure
EFFECT OF CHANGE OF TEMPERATURE
On increasing the temperature of solids,
the kinetic energy of the particles increases.
Due to the increase in kinetic energy, the
particles start vibrating with greater speed.
The energy supplied by heat overcomes the
forces of attraction between the particles. The
particles leave their fixed positions and start
moving more freely.
A stage is reached when
the solid melts and is converted to a liquid.
👉The minimum temperature at which a solid
melts to become a liquid at the atmospheric
pressure is called its melting point. The melting point of ice is 273.15 K.
👉The
process of melting, that is, change of solid
state into liquid state is also known as fusion
When a solid melts, its temperature
remains the same, so where does the heat
energy go?
Heat gets used up in changing the state by overcoming the forces of attraction
between the particles. As this heat energy is
absorbed by ice without showing any rise in
temperature, it is considered that it gets
hidden into the contents of the beaker and is
known as the latent heat.
Latent Heat of Fusion
👉The amount of heat energy
that is required to change 1 kg of a solid into
liquid at atmospheric pressure at its melting
point is known as the latent heat of fusion.
👉The temperature at which a liquid
starts boiling at the atmospheric pressure is
known as its boiling point.
For water this temperature is `373 K` `(100 ℃ = 273 + 100 = 373 K)`.
Sublimation
👉Sublimation is the change of solid state directly to gaseous
state without going through liquid state.
Deposition
👉Deposition is the change of gaseous state directly to solid
state without going through liquid state.
EFFECT OF CHANGE OF PRESSURE
Applying pressure and reducing
temperature can liquefy gases.
Solid carbon dioxide `(CO_2
)` is stored under high pressure. Solid `CO_2` gets converted directly to gaseous state
on decrease of pressure to 1 atmosphere without coming into liquid state. This is the
reason that solid carbon dioxide is also known
as dry ice.
Evaporation
In the case of liquids, a small
fraction of particles at the surface, having
higher kinetic energy, is able to break away
from the forces of attraction of other particles
and gets converted into vapour.
Evaporation is a surface phenomenon.
👉 The process of a liquid changing into vapour even below its boiling point is called evaporation.
Factors Affecting Evaporation
1. Temperature
2. Surface area
3. Humidity
4. Wind speed
COOLING CAUSED BY EVAPORATION
The cooling caused by evaporation is based on the fact that when a liquid evaporates, it draws the latent heat of vaporisation from anything that it touches. By losing heat, anything gets cooled.
The particles of liquid absorb
energy from the surroundings. This
absorption of energy from the surroundings
makes the surroundings cold.
When you pour some
acetone (nail polish remover) on your palm. The particles gain energy from your palm or
surroundings and evaporate causing the
palm to feel cool
Why should we wear cotton clothes in summer?
During summer, we perspire more
because of the mechanism of our body which
keeps us cool. During
evaporation, the particles at the surface of
the liquid gain energy from the surroundings
or body surface and change into vapour. The
heat energy equal to the latent heat of
vaporisation is absorbed from the body
leaving the body cool. Cotton, being a good
absorber of water helps in absorbing the
sweat and exposing it to the atmosphere for
easy evaporation.
Why do we see water droplets on the outer surface of a glass containing ice-cold water?
The water
vapour present in air, on coming in contact
with the cold glass of water, loses energy and
gets converted to liquid state, which we see
as water droplets.
References
- Foundation Science Chemistry Class IX
- NCERT Science Class IX
- Chemistry Class IX (S. Chand)
Tags:
Class IX





Nice notes to better understand 😊👍👌👍👌👌☺️
ReplyDeleteThanks
ReplyDeleteVery interesting notes👌👌👌
ReplyDeleteThank you
Delete👍👆👆👆👌 excellent notes ☺️
ReplyDeleteVery impressive
ReplyDelete“It is really easy, and nice
ReplyDelete👆💥🎉🎉 awesome 📚h👍👍👍
ReplyDeleteThank you
DeleteNice 👏notes📝 and video 👌👌👌👌
ReplyDeleteGourav
ReplyDeleteVery good👍👍👍 notes📝 hi Sir
Thanks, Gaurav!!
DeleteGourav Kumar
ReplyDeleteNice👏👍👏👍 notes📝 Hi Sir
Waa h👩🏫 u are amazing 🎉🎉👍👍👍👍👍👍👍
ReplyDeleteYou're the best teacher ever👍👍👍👍👍👩🏫
ReplyDeleteThank you so much
Delete