📝 Acids, Bases, and Salts
The elements combine to form a large number of compounds. Based on their chemical properties, all the compounds can be classified into three groups:
1) Acids
2) Bases
3) Salts
Indicators for testing acids and bases
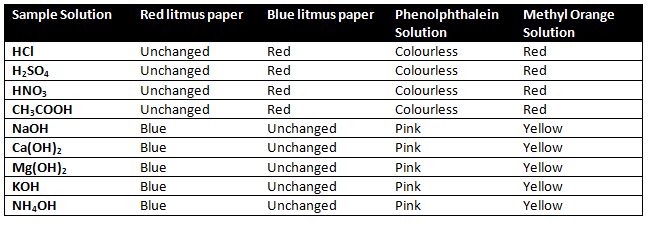
The three most common indicators are Litmus, Methyl orange, and Phenolphthalein.
Litmus paper 👉 Blue litmus and Red litmus.
1) An acid turns blue litmus to red
2) A base turns red litmus to blue.
Methyl orange 👉The color of methyl orange is orange.
1) Methyl orange indicator gives red color in
acid solution.
2) Methyl orange indicator gives the yellow color
in the basic solution.
Phenolphthalein is colourless.
1) Phenolphthalein indicator is colourless in an acidic solution.
2) Phenolphthalein indicator gives pink colour in basic solution.
Understanding the chemical properties of acids and bases
- Acids and bases in the laboratory
- How do acids and bases react with metals?
- How do metal carbonates and metal hydrocarbonates react with acids?
- How do acids and bases react with each other?
- Reaction of metallic oxides with acids
- Reaction of non-metallic oxide with base
1) Acids and bases in the laboratory
What change in colour did you observe with red litmus, blue litmus, phenolphthalein, and methyl orange solutions for each of the solutions taken?
Olfactory indicators
There are some substances whose odour changes in acidic or basic media. These are called olfactory indicators. Examples: Cloth strip treated with onion, dilute vanilla essence, and clove oil.
Onion has a characteristic smell. When a basic solution like sodium hydroxide solution is added to a cloth strip treated with onion, then onion smell cannot be detected. An acidic solution like hydrochloric acid, however, does not destroy the smell of onion.
Dilute vanilla essence + `HCl` ➠ Smell remained
Dilute vanilla essence + `NaOH` ➠ Smell removed
Clove oil + `HCl` ➠ Smell remained
Clove oil + NaOH ➠ Smell removed
Conclusions
⇓
- Olfactory indicators do not lose their smell in acidic media.
- Olfactory indicators lose their smell basic media.
2) How do acids and bases react with metals?
Experiment 🔎
► Take about 5 mL of dilute sulphuric acid in a test tube and add a few pieces of zinc granules to it.
► We will observe the formation of gas bubbles on the surface of zinc granules.
► Pass the gas being formed through the soap solution taken in a trough (by the means of a glass delivery tube). Gas-filled bubbles are formed in the soup solution which rises into the air.
►Bring a burning candle near a glass-filled soap bubble. The gas present in the soap bubble burns with a 'pop' sound (making a little explosion ).
► Only hydrogen gas burns making a 'pop' sound. This shows that hydrogen gas is evolved in the reaction of dilute sulphuric acid with zinc.
`Zn + H_2SO_4 \rightarrow ZnSO_4 + H_2`
(Zinc + Sulphuric acid `\rightarrow` Zinc Sulphate+ Hydrogen)
Note that the metal in the above reactions displaces hydrogen atoms from the acids as hydrogen gas and forms a compound called salt. Thus, the reaction of a metal with an acid can be summarised as –
Acid + Metal `\rightarrow` Salt + Hydrogen gas
The metal reacts with a base
`NaOH + Zn \rightarrow Na_2ZnO_2 + H_2`
(Sodium hydroxide + Zinc `\rightarrow` Sodium Zincate + Hydrogen )
You find again that hydrogen is formed in the reaction. However, such reactions are not possible with all metals.
3) How do metal carbonates and metal hydrocarbonates react with acids?
Experiments 🔎
► Take two test tubes, label them as A and B.
► Take about 0.5 g of sodium carbonate `(Na_2CO_3)` in test tube A and about 0.5 g of sodium hydrogen carbonate `(NaHCO_3)` in test tube B.
Reactions
➤ Test tube A: `Na_2CO_3 + 2HCl \rightarrow 2NaCl + H_2O + CO_2`
➤ Test tube B: `NaHCO_3 + HCl \rightarrow NaCl + H_2O + CO_2`
➤ On passing the carbon dioxide gas evolved through lime water, the lime water turns milky due to the formation of a white precipitate of calcium carbonate
`Ca(OH)_2 + CO_2 \rightarrow CaCO_3 + H_2O`
[Calcium hydroxide + Carbon dioxide `\rightarrow` Calcium carbonate ( white ppt.) + Water]
➤ On passing excess carbon dioxide the following reaction takes place, the white precipitate formed first dissolves due to the formation of a soluble salt calcium hydrogen carbonate.
`CaCO_3 + H_2O + CO_2 \rightarrow Ca(HCO_3)_2`
(Calcium carbonate + Water + Carbon diaixide `\rightarrow` Calcium hydrogen carbonate)
All metal carbonates and hydrogen carbonates react with acids to give a corresponding salt, carbon dioxide, and water. Thus, the reaction can be summarised as –
Metal carbonate/Metal hydrogencarbonate + Acid `\rightarrow` Salt + Carbon dioxide + Water
Please note that limestone, marble, and chalk are the different forms of the same compound of calcium carbonate.
4) How do acids and bases react with each other?
Acid + Base `\rightarrow` Salt + Water
Experiments 🔍
► Take about 2 mL of dilute NaOH solution in a test tube and add two drops of phenolphthalein solution.
► The colour of the solution becomes pink.
► Add dilute `HCl` solution to the above solution drop by drop.
► After adding a certain volume of `HCl` we find that the pink colour just disappears.
When an acid is treated with a base, the base neutralises the acid and destroys its acidity, so the reaction between an acid and a base to form salt and water is called a neutralisation reaction.
`NaOH + HCl \rightarrow NaCl + H_2O`
(Sodium hydroxide + Hydrochloric acid `\rightarrow` Sodium Chloride + Water)
5) Reaction of metallic oxides with acids
Metal oxide + Acid `\rightarrow` Salt + Water
Experiments
► Take a small amount of copper oxide (Black in colour ) in a beaker and add dilute hydrochloric acid slowly while stirring.
► The colour of the solution becomes blue-green and the copper oxide dissolves.
The blue-green colour of the solution is due to the formation of copper(II) chloride in the reaction.
`CuO + 2HCl \rightarrow CuCl_2 + H_2O`
[Copper oxide (Black) + Hydrochloric acid `\rightarrow` Copper choride (Blue- green) + Water]
Since metallic oxides react with acids to give salts and water, similar to the reaction of a base with an acid, metallic oxides are said to be basic oxides. It shows the basic nature of metal oxide.
6) Reaction of non-metallic oxide with base
Base + Non-metal oxide `\rightarrow` Salt + Water
`Ca(OH)_2 + CO_2 \rightarrow CaCO_3 + H_2O`
[Calcium hydroxide + Carbon dioxide `\rightarrow` Calcium Carbonate (Salt) + Water]
The reactions of non-metal oxides with bases to form salt and water show that non-metal oxides are acidic in nature.
WHAT DO ALL ACIDS AND ALL BASES HAVE IN COMMON?
All acids contain hydrogen. The hydrogen present in acids in such that when acid is dissolved in water, it separates out as positively charged hydrogen ions `(H^+)` and enters the solution as `H^+`(aq) ions.
➤ An acid is a substance that dissociates (or ionises) on dissolving in water to produce hydrogen ions `[H^+ (aq)]`
`HCl (aq) \rightarrow H^+ (aq) + Cl^- (aq)`
Please note that hydrogen ions do not exist as `H^+` ions in solution, they attach themselves to the polar water molecules to form hydronium ions, `H_3O^+`.
`H^+ + H_2O \rightarrow H_3O^+`
So, hydrogen ions must always be written as either `H^+` (aq) or as hydronium ions, `H_3O^+`. `H^+` (aq) and `H_3O^+` are the same.
Experiments 🔍
Acid solution in water conducts electricity
● Take solutions of glucose, alcohol, hydrochloric acid, sulphuric acid, etc.
● Fix two nails on a cork, and place the cork in a 100 mL beaker.
● Connect the nails to the two terminals of a 6-volt battery through a bulb and a switch
● Now pour some dilute HCl into the beaker and switch on the current.
● Repeat with dilute sulphuric acid.
● Repeat the experiment separately with glucose and alcohol solutions. What do you observe now?
● Does the bulb glow in all cases?
The bulb will start glowing in the case of acids, as shown in Fig. But you will observe that glucose and alcohol solutions do not conduct electricity. The glowing of the bulb indicates that there is a flow of electric current through the solution. The electric current is carried through the acidic solution by ions.
Acids contain `H^+` ion as cation and anion such as `Cl^–` in `HCl`, `NO_3^ –` in `HNO_3, SO_4^{2- }` in `H_2SO_4, CH_3COO^–` in `CH_3COOH`. Since the cation present in acids is `H^+`, this suggests that acids produce hydrogen ions, `H^+`(aq), in solution, which are responsible for their acidic properties.
What happens when bases are dissolved in water?
`NaOH (S) \overset {H_2O} \rightarrow Na^+ (aq) + OH^-`(aq)
`KOH (S) \overset {H_2O} \rightarrow K^+(aq) + OH^-`(aq)
`Mg(OH)_2 (S) \overset {H_2O} \rightarrow Mg^{2+}(aq) + 2OH^-`(aq)
Bases generate hydroxide `(OH^–)` ions in water. Bases that are soluble in water are called alkalis.
Experiments 🔍
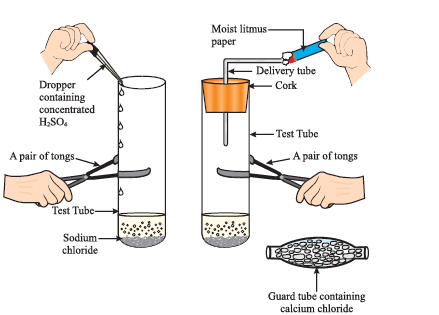
● Take about 1g solid NaCl in a clean and dry test tube and set up the apparatus as shown in Fig.
Preparation of HCl gas
● Add some concentrated sulphuric acid to the test tube.
● What do you observe? Is there gas coming out of the delivery tube?
● Test the gas evolved successively with dry and wet blue litmus paper.
● In which case does the litmus paper change colour?
● On the basis of the above Activity, what do you infer about the acidic character of:
(i) dry HCl gas
(ii) HCl solution?
●If the climate is very humid, you will have to pass the gas produced through a guard tube (drying tube) containing calcium chloride to dry the gas.
`NaCl (s) + H_2SO_4 (aq) \rightarrow NaHSO_4 (aq) + HCl (g)`
➤This experiment suggests that hydrogen ions in HCl are produced in the presence of water. The separation of H+ ions from HCl molecules can not occur in the absence of water.
`HCl + H_2O \rightarrow H_3O^+ + Cl^–`
➤Hydrogen ions cannot exist alone, but they exist after combining with water molecules. Thus hydrogen ions must always be shown as
`H^+(aq)` or hydronium ion `(H_3O^+)`.
Experiments 🔍
● Take 10 mL water in a beaker
● Add a few drops of concentrated `H_2SO_4` to it and swirl the beaker slowly.
● Touch the base of the beaker.
● Is there a change in temperature?
● Is this an exothermic or endothermic process?
● Repeat the above Activity with sodium hydroxide pellets.
The process of dissolving an acid or a base in water is a highly exothermic one. Care must be taken while mixing concentrated nitric acid or sulphuric acid with water. The acid must always be added slowly to water with constant stirring.
If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns. The glass container may also break due to excessive local heating. Look out for the warning sign (shown in Fig.) on the can of concentrated sulphuric acid and on the bottle of sodium hydroxide pellets.
Warning sign displayed on containers containing concentrated acids and bases
➤ Mixing an acid or base with water results in a decrease in the concentration of ions `(H_3O^+ or OH^–)` per unit volume. Such a process is called dilution and the acid or the base is said to be diluted.
How strong are acid or base solutions?
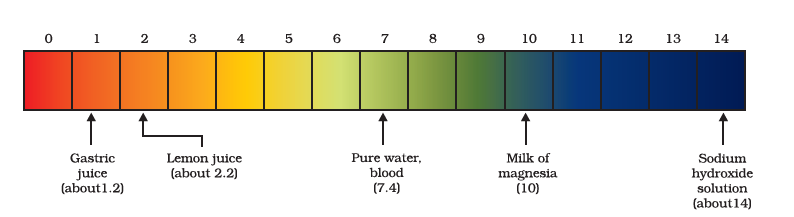
Can we quantitatively find the amount of these ions present in a solution? Can we judge how strong a given acid or base is? We can do this by making use of a universal indicator. The universal indicator shows different colours at different concentrations of hydrogen ions in a solution.
A scale for measuring hydrogen ion concentration in a solution, called pH scale. The p in pH stands for ‘potenz’ in German, meaning power. On the pH scale, we can measure pH generally from 0 (very acidic) to 14 (very alkaline). pH should be thought of simply as a number that indicates the acidic or basic nature of a solution. The higher the hydronium ion concentration, the lower is the pH value.
The pH of a neutral solution is 7. Values less than 7 on the pH scale represent an acidic solution. As the pH value increases from 7 to 14, it represents an increase in `OH^–` ion concentration in the solution, that is, increase in the strength of alkali. Generally, paper impregnated with the universal indicator is used for measuring pH.
Variation of pH with the change in concentration of `H^+(aq)` and `OH^–(aq)` ions
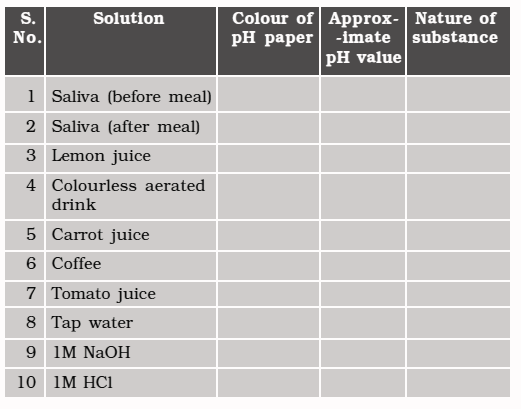
Experiments 🔎
● Test the pH values of solutions given in Table.
● Record your observations.
pH of some common substances shown on a pH paper
The strength of acids and bases depends on the number of `H^+` ions and `OH^–` ions produced, respectively. If we take hydrochloric acid and acetic acid of the same concentration, then these produce different amounts of hydrogen ions. Acids that give rise to more `H^+` ions are said to be strong acids, and acids that give less `H^+` ions are said to be weak acids.
Importance of pH in everyday life.
Are plants and animals pH sensitive?
Our body works within the pH range of 7.0 to 7.8. Living organisms can survive only in a narrow range of pH changes. When pH of rainwater is less than 5.6, it is called acid rain. When acid rain flows into the rivers, it lowers the pH of the river water. The survival of aquatic life in such rivers becomes difficult.
What is the pH of the soil in your backyard?
Plants require a specific pH range for their healthy growth. For most plants, the optimum pH range is from 5.5 to 7.0, but some plants will grow in more acid soil or may require a more alkaline level.
pH in our digestive system
It is very interesting to note that our stomach produces hydrochloric acid (The pH is 1.5 to 3.5). It helps in the digestion of food without harming the stomach. During indigestion, the stomach produces too much acid and this causes pain and irritation. To get rid of this pain, people use bases called antacids. These antacids neutralize the excess acid. Magnesium hydroxide (Milk of magnesia), a mild base, is often used for
this purpose.
pH change as the cause of tooth decay
Tooth decay starts when the pH of the mouth is lower than 5.5. Tooth enamel, made up of calcium hydroxyapatite (a crystalline form of calcium phosphate) is the hardest substance in the body. It does not dissolve in water but is corroded when the pH in the mouth is below 5.5. Bacteria present in the mouth produce acids by degradation of sugar and food particles remaining in the mouth after eating. The best way to prevent this is to clean the mouth after eating food. Using toothpaste, which is generally basic, for cleaning the teeth can neutralize the excess acid and prevent tooth decay.
Salts
Salts are formed when acids react with bases. The name of a salt consist of two parts: the first part of the name of salt is derived from the name of base, and the second part of the name of the salt comes from the name of acid. For example, the name of a salt called 'sodium chloride' (`NaCl`) comes from sodium hydroxide (`NaOH`) base and hydrochloric acid (`HCl`). Please note that:
(i) The salts of 'hydrochloric acid' are called 'chlorides.'
(ii) The salts of 'sulphuric acid' are called 'sulphates.'
(iii) The salts of 'nitric acid' are called 'nitrates.'
(iv) The salts of 'carbonic acid' are called 'carbonates.'
(v) The salts of 'acetic acid' are called 'acetates.'
Family of Salts
The salts having the same positive ions (or same negative ions) are said to belong to a family of salts. For example, sodium chloride (`NaCl`) and sodium sulphate (`Na_2SO_4`) belongs to same family of salts called 'sodium salts'. (because they both contain the same positive ions, sodium ions, `Na^+`). Similarly sodium chloride (`NaCl`) and potassium chloride (`KCl`) belong to the same family of salts called 'chloride salts'.
The pH of Salt Solutions
➤ Salts of a strong acid and a strong base are neutral with pH value of 7
`NaOH + HCl rightarrow NaCl + H_2O`
➤ Salts of a strong acid and weak base are acidic with pH value less than 7
`NH_4OH + HCl rightarrow NH_4Cl + H_2O`
➤ Salts of a strong base and weak acid are basic in nature, with pH value more than 7.
`2NaOH + H_2CO_3 rightarrow Na_2CO_3 + 2H_2O`
Common Salts ( Sodium chloride)
Sodium chloride can be made in the laboratory by the combination of sodium hydroxide and hydrochloric acid
`NaOH + HCl rightarrow NaCl + H_2O`
But sodium chloride is never made in this way on a large scale because it is present in nature in abundance.
How common salt is obtained?
(i) Common salt is from seawater
(ii) Common salt from underground deposits
(i) Common salt is from seawater
Common salt is obtained from seawater by the process of evaporation. Seawater is trapped in a large, shallow pool and allowed to stand there. The sun's heat evaporates the water slowly and common salt is left behind.
(ii) Common salt from underground deposits
The large crystals of common salt found in underground deposits are called rock salt. Rock salt is mined from the underground deposits just like a coil.
Uses of common salts
(i) It is used in cooking food. It improves the flavour of food.
(ii) Common salt is used as a preservative in pickles.
(iii) Sodium chloride is used in the manufacturing of soap.
(iv) Common salt is used as raw material in the industry such as sodium hydroxide (caustic soda), sodium carbonate (washing soda), sodium hydrogen carbonate (baking soda), etc.
Baking soda
The baking soda is commonly used in the kitchen for making tasty crispy pakoras, etc. Sometimes it is added for faster cooking. The chemical name of the compound is sodium hydrogen carbonate (`NaHCO_3`). It is produced using sodium chloride as one of the raw materials.
`NaCl + H_2O + CO_2 + NH_3 rightarrow NH_4Cl + NaHCO_3`
`NaHCO_3` is a mild non-corrosive base. The following reaction takes place when it is heated during cooking `2NaHCO_3 overset {triangle}rightarrow Na_2CO_3 + H_2O + CO_2`
Uses of Baking soda
(i) Sodium hydrogen carbonate is used as an antacid in medicine to remove the acidity of the stomach.
(ii) Baking soda is used in making baking powder (used in making cake, bread, etc.). Baking powder is a mixture of baking soda and mild edible acid such as tartaric acid.
`NaHCO_3 (aq) + H^+ (aq) rightarrow Na^+ (aq) + CO_2 (g) + H_2O (l)`
(Sodium hydrogen carbonate + Hydrogen ions (from tartaric acid) `rightarrow` Sodium ions (from sodium tartaric salt) + Carbon dioxide + Water)
The carbon dioxide gas produced gets trapped in the wet dough and bubbles out slowly making the cake rise and become soft and spongy. Baking soda is a single compound: sodium hydrogen carbonate. On the other hand baking powder is a mixture of sodium hydrogen carbonate and an edible acid such as tartaric acid or citric acid.
(iii) It is also used in soda-acid fire extinguishers.
Soda-acid-type fire extinguishers contain solutions of sodium hydrogen carbonate and sulphuric acid in separate containers inside them.
 |
| source: S.chand Chemistry |
Washing Soda
Washing soda is sodium carbonate containing 10 molecules of water of crystallisation. That is washing soda is sodium carbonate decahydrate. The formula of washing soda is `Na_2CO_3 .10H_2O`. Sodium carbonate which does not contain any water of crystallisation is called anhydrous sodium carbonate, `Na_2CO_3`. Anhydrous sodium carbonate, `Na_2CO_3` is commonly known as soda ash. Washing soda is an important chemical obtained from sodium chloride.
Production of Washing Soda
Washing soda is produced from sodium chloride in the following three steps:
(i) A concentrated solution of sodium chloride (brine) is reacted with ammonia and carbon dioxide to obtain sodium hydrogen carbonate.
`NaCl + H_2O + CO_2 + NH_3 rightarrow NH_4Cl + NaHCO_3`
Sodium hydrogen carbonate formed is slightly soluble in water, so it precipitates out as solid.
(ii) Sodium hydrogen carbonate is separated by filtration, dried, and heated. On heating, sodium hydrogen carbonate decomposes to form sodium carbonate.
`2NaHCO_3 overset {triangle}rightarrow Na_2CO_3 + H_2O + CO_2`
Anhydrous sodium carbonate obtained here is called soda ash.
(iii) Anhydrous sodium carbonate (soda ash ) is dissolved in water and recrystallization to get washing soda crystals containing 10 molecules of water of crystallisation.
`Na_2CO_3 + 10H_2O rightarrow Na_2CO_3 .10H_2O`
Uses of washing soda
(i) Sodium carbonate (washing soda) is used in glass, soap, and paper industries.
(ii) It is used in the manufacture of sodium compounds such as borax.
(iii) Washing soda can be used as a cleaning agent for domestic purposes.
(iv) It is used for removing the permanent hardness of the water.
Are the crystals of salts really dry?
On heating the copper sulpahte crystals, water droplets come. Copper sulphate crystals that seem to be dry contain water of crystallisation. When we heat the crystals, this water is removed and the salt turns white.
Crystals of `CuSO_4. 5H_2O`
 |
| https://en.wikipedia.org/wiki/Copper(II)_sulfate |
If you moisten the crystals again with water, you will find that the blue colour of the crystals reappears.
Water of crystallisation is the fixed number of water molecules present in one formula unit of salt. Five water molecules are present in one formula unit of copper sulphate. The chemical formula for hydrated copper sulphate is `Cu SO_4. 5H_2O`.
One other salt, which possesses water of crystallisation is gypsum. It has two water molecules as water of crystallisation. It has the chemical formula `CaSO_4. 2H_2O`.
Water of Crystallisation: Hydrated Salts
There are some salts that contain a few water molecules as an essential part of their crystals structure. The water molecules which form part of the structure of crystals (of salts) are called water of crystallisation. The salts which contain water of crystallisation are called hydrated salts. Every hydrated salt has a fixed number of molecules of water of crystallisation in its one formula unit.
Examples
1. Copper sulphate crystals contains 5 molecules of water of crystallisation in one formula unit and hence written as `CuSO_4. 5H_2O`. It is called copper sulphate pentahydrate.
2. `Na_2CO_3. 10H_2O` (Sodium carbonate decahydrate).
3. `FeSO_4. 7H_2O` (Iron sulphate heptahydrate).
It should be noted that the water of crystallisation is a part of the crystals structure of the salt. Since water of crystallisation is not free water, it does not wet the salt. The water of crystallisation gives the crystals of salts their shape and colour.
Action of heat on hydrated salts
When hydrated salts are heated strongly, they lose their water of crystallization. By losing water of crystallisation, the hydrated salts lose their regular shape and colour, and become powdery substances. The salts which have lost their water of crystallisation are called anhydrous salts. When water is added to an anhydrous salt, it becomes hydrated once again, and regain its colour.
Examples
On strong heating, blue copper sulphate crystals (copper sulphate pentahydrate) turn white, due to the loss of water of crystallisation.
`CuSO_4. 5H_2O overset{triangle}{rightarrow} CuSO_4 + 5H_2O`
Anhydrous copper sulaphte turns blue on adding water.
`CuSO_4 + 5H_2O rightarrow CuSO_4. 5H_2O`
Plaster of Paris
Plaster of Paris is calcium sulphate hemihydrate (calcium sulphate half-hydrate). The formula of plaster of Paris is `CaSO_4. 1/2H_2O`.
On heating gypsum at 373K, it loses water molecules and becomes calcium sulphate hemihydrate
`CaSO_4. 2H_2O overset {373K} rightarrow CaSO_4. 1/2H_2O + 1 1/2H_2O`
(Gypsum `overset {373K} rightarrow` Paster of Paris + Water)
If gypsum is heated above `100^°`C (373K), then the water of crystallisation is eliminated and anhydrous calcium sulphate called dead burnt plaster is formed.
The formula `CaSO_4. 1/2H_2O` actually means that, two formula units of `CaSO_4` share one molecule of water. It can also be written as `2CaSO_4. H_2O`.
Plaster of Paris is a white powder and on mixing with water, it changes to gypsum once again giving a hard solid mass, which doctors use as plaster for supporting fractured bones in the right position.
`CaSO_4. 1/2H_2O + 1 1/2H_2O rightarrow CaSO_4. 2H_2O`
Uses of Plaster of Paris
(i) Plaster of Paris is used in hospitals for setting fractured bones in the right position.
(ii) It is used in making toys, decorative materials, cheap ornaments, cosmetics, etc.
Sodium Hydroxide
Sodium hydroxide is commonly known as caustic soda. The chemical formula of sodium hydroxide is NaOH.
Production of Sodium Hydroxide
Sodium hydroxide is produced by the electrolysis of a concentrated aqueous solution of sodium chloride (brine).
When electricity is passed through a concentrated solution of sodium chloride (brine), it decomposes to form sodium hydroxide, chlorine, and hydrogen.
Chlor-alkali process
During electrolysis, chlorine gas is produced at the anode (positive electrode) and hydrogen gas is produced at the cathode (negative electrode). Sodium hydroxide is formed near the cathode. This process of electrolysis of sodium chloride solution is called the Chlor-alkali process because of the product formed: chlor for chlorine and alkali for sodium hydroxide.
Uses of Sodium Hydroxide
1. It is used for making soaps and detergent.
2. Sodium hydroxide is used for making artificial textile fibres (rayon).
3. Sodium hydroxide is used in purifying bauxite ore from which aluminium metal is extracted.
4. It is used in degreasing metals, oil refining, and making dyes and bleaches.
Summary
● Acid-base indicators are dyes or mixtures of dyes that are used to indicate the presence of acids and bases.
● The acidic nature of a substance is due to the formation of `H^+(aq)` ions in the solution. The formation of `OH^–(aq)` ions in solution is responsible for the basic nature of a substance.
● When an acid reacts with a metal, hydrogen gas is evolved and a corresponding salt is formed.
● When a base reacts with a metal, along with the evolution of hydrogen gas a salt is formed which has a negative ion composed of the metal and oxygen.
● When an acid reacts with a metal carbonate or metal hydrogen carbonate, it gives the corresponding salt, carbon dioxide gas, and water.
● Acidic and basic solutions in water conduct electricity because they produce hydrogen and hydroxide ions respectively.
● The strength of an acid or an alkali can be tested by using a scale called the pH scale (0-14) which gives the measure of hydrogen ion concentration in a solution.
● A neutral solution has a pH of exactly 7, while an acidic solution has a pH less than 7 and a basic solution has a pH of more than 7.
● Living beings carry out their metabolic activities within an optimal pH range.
● Mixing concentrated acids or bases with water is a highly exothermic process.
● Acids and bases neutralise each other to form corresponding salts and water.
● Water of crystallisation is the fixed number of water molecules present in one formula unit of salt.
● Salts have various uses in everyday life and in industries.
work in progress
References
- NCERT Science Class 10
- S. Chand Chemistry Class 10
- https://en.wikipedia.org/wiki/Copper(II)_sulfate









Very Nice
ReplyDeleteThanks, dear.
DeleteGood 👍👍👍👍
ReplyDeleteThanks, dear
DeleteThis comment has been removed by the author.
ReplyDeleteNice notes
ReplyDeleteThank you
Deletenotes for best marks
ReplyDeleteThanks sir
🤘👌👌👌👌
ReplyDeleteGourav Kumar
ReplyDeleteVery Nice notes 👍👍sir
Your Good channel and website
Outstanding note
ReplyDeleteThanks, you!!
Delete👍👍👍👍👍👍🙂
ReplyDeleteWell done great job 🎉🎉🎉🎉
Thank you!!
DeleteWow very good notes☺thanks for providing
ReplyDeleteThank you
DeleteBest and Most useful notes in whole Google... Thank you Jay sir for providing this kind of useful content to us...☺️
ReplyDeleteThank you
Deleteif you have to put someone on a pedestal put teachers. they are society's heroes
ReplyDeleteThank you
DeleteTeaching is the greatest act of optimism. ...🙂🙂
ReplyDeleteBest and most useful notes in whole other websites
ReplyDeleteWow sir you are really very great teache
ReplyDelete