Periodic Classification of Elements
Introduction
Scientists have discovered 118 elements. Some of these elements occur in a free state and some in a combined state. When very few elements were known, studying them separately was not a problem. But when a large number of elements had been discovered, it becomes difficult to study the properties of all of them separately. So, attempts were made to sort out the elements to groups to follow their behaviour in an orderly manner. The study of the properties of typical elements of a particular group enables scientists to predict roughly the properties of the other elements of that group.
Dobereiner's Classification
In 1817, German chemist Johann Dobereiner classified elements having similar chemical properties into groups of three. These groups of three elements were called triads.
Dobereiner's law of triads.
According to this law, when elements are arranged in the order of increasing atomic mass in a triad, the atomic mass of the middle element was found to be approximately equal to the arithmetic mean of the atomic masses of the other two elements.
|
Element |
Atomic mass |
Element |
Atomic mass |
Element |
Atomic mass |
|
Calcium |
40 |
Lithium |
7 |
Chlorine |
35.5 |
|
Strontium |
87.5 |
Sodium |
23 |
Bromine |
80 |
|
Barium |
137 |
Potassium |
39 |
Iodine |
127 |
|
Arithmetic mean of calcium & barium = |
40+137/2 = 88.5 |
Arithmetic mean of lithium & potassium = |
7+39/2 = 23 |
Arithmetic mean of chlorine & iodine = |
35.5+127/2 = 81.2 |
|
Atomic mass of strontium = 87.5 |
|
Atomic mass of sodium = 23 |
|
Atomic mass of bromine = 80 |
|
The classification of elements into triads was very successful in predicting the atomic mass and properties of the middle element.
Limitation
The limitation was that it failed to arrange all the known elements in the form of triads of elements having similar chemical properties. Dobereiner could identify only three triads from the elements known at that time.
Newland's Classification (Newland's Law of Octaves)
Law of octaves, in 1864, John Newlands, an English chemist, showed that when elements are arranged in the order of their increasing atomic masses, the eighth elements, starting from a given element, was a kind of repetition of the first one, like the eighth note in an octave of music, i.e.,
sa, re, ga, ma, pa, dha, ni, sa
where the first note and the eighth note are the same
|
sa (do) |
re (re) |
ga (mi) |
ma (fa) |
pa (so) |
dha (la) |
ni (ti) |
|
H |
Li |
Be |
B |
C |
N |
O |
|
F |
Na |
Mg |
Al |
Si |
P |
S |
|
Cl |
K |
Ca |
Cr |
Ti |
Mn |
Fe |
|
Co & Ni |
Cu |
Zn |
Y |
In |
As |
Se |
|
Br |
Rb |
Sr |
Ce & La |
Zr |
- |
- |
When elements are arranged in the order of increasing atomic masses, the properties of the eighth element are a repetition of the properties of the first element.
In the Indian system of music, there are seven musical notes on a scale – sa, re, ga, ma, pa,
dha, ni. In the west, they use the notations – do, re, mi, fa, so, la, ti. The notes in a scale are
separated by whole and half-step frequency intervals of tones and semitones. A musician
uses these notes for composing the music of a song. Naturally, there must be some repetition
of notes. Every eighth note is similar to the first one and it is the first note of the next scale.
Starting from lithium (Li) the eighth element is sodium (Na). The eighth element starting from sodium is potassium. The properties of lithium, sodium, and potassium are similar. The properties of beryllium, magnesium, and calcium are similar too.
Limitations of Newland's Law of Octaves
1. It was found that the Law of Octaves was applicable only up to calcium, as after calcium every eighth element did not possess properties similar to that of the first.
2. It was assumed by Newlands that only 56 elements existed in nature and no more elements would be discovered in the future. But, later on, several new elements were discovered, whose properties did not fit into the Law of Octaves.
3. In order to fit elements into his Table, Newlands adjusted two elements in the same slot, but also put some unlike elements under the same note. Note that cobalt and nickel are in the same slot and these are placed in the same column as fluorine, chlorine, and bromine which have very different properties than these elements. Iron, which resembles cobalt and nickel in properties, has been placed far away from these elements. Thus, Newlands’ Law of Octaves worked well with lighter elements only.
Mendeléev Periodic Table
When Dmitri Ivanovich Mendeléev started his work, 63 elements were known. He examined the relationship between the atomic masses of the elements and their physical and chemical properties. Among chemical properties, Mendeléev concentrated on the compounds formed by elements with oxygen and hydrogen. He selected hydrogen and oxygen as they are very reactive and formed compounds with most elements. The formulae of the hydrides and oxides formed by an element were treated as one of the basic properties of an element for its classification. He then took 63 cards and on each card he wrote down the properties of one element. He observed that most of the elements got a place in a Periodic Table and were arranged in the order of their increasing atomic masses. It was also observed that there occurs a periodic recurrence of elements with similar physical and chemical properties. On this basis, Mendeléev formulated a Periodic Law, which states that ‘the properties of elements are the periodic function of their atomic masses’.
Mendeléev’s Periodic Table contains vertical columns called ‘groups’ and horizontal rows called ‘periods’. Mendeléev’s Periodic Law means that if elements are arranged in order of increasing atomic masses, then the properties of elements are repeated after regular intervals or periods
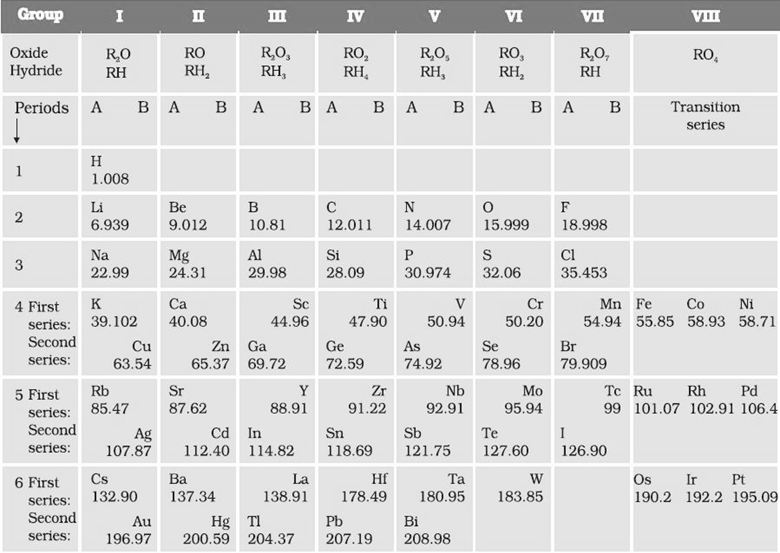
Mendeléev’s Periodic Table
Achievements of Mendeléev’s Periodic Table
While developing the Periodic Table, there were a few instances where Mendeléev had to place an element with a slightly greater atomic mass before an element with a slightly lower atomic mass. The sequence was inverted so that elements with similar properties could be grouped together. For example, cobalt (atomic mass 58.9) appeared before nickel (atomic mass 58.7). Can you find out one more such anomaly?
Further, Mendeléev left some gaps in his Periodic Table. Instead of looking upon these gaps as defects, Mendeléev boldly predicted the existence of some elements that had not been discovered at that time. Mendeléev named them by prefixing a Sanskrit numeral, Eka (one) to the name of the preceding element in the same group. For instance, scandium, gallium, and germanium discovered later, have properties similar to Eka–boron, Eka–aluminium, and Eka–silicon, respectively. The properties of Eka–Aluminium predicted by Mendeléev and those of the element, gallium which was discovered later and replaced Eka-aluminium, are listed as follows
Properties of Eka- aluminium and gallium
We can see from the table that the properties of eka- aluminium predicted by Mendeleev are almost exactly the same as the actual properties of the gallium element. Before the discovery, it was called eka- aluminium and after discovering it became gallium.
When a whole new group of elements called noble gases was discovered, it got a place in the periodic table in the form of a separate group. It did not disturb the original arrangement of Mendeleev's periodic table.
Limitations of Mendeleev's Classification
The electronic configuration of hydrogen resembles that of alkali metals. Like alkali metals, hydrogen combines with halogens, oxygen, and sulphur to form compounds having similar formulae, as shown in examples here.
On the other hand, just like halogens, hydrogen also exists as diatomic molecules and it combines with metals and non-metals to form covalent compounds.
Certainly, no fixed position can be given to hydrogen in the Periodic Table. This was the first limitation of Mendeléev’s Periodic Table. He could not assign a correct position to hydrogen in his Table.
Isotopes of all elements posed a challenge to Mendeleev’s Periodic Law. Another problem was that the atomic masses do not increase in a regular manner in going from one element to the next. So it was not possible to predict how many elements could be discovered between two elements especially when we consider the heavier elements.
Modern Periodic Table
In 1913, Henry Moseley showed that the atomic number of an element is a more fundamental property than its atomic mass. Accordingly, Mendeléev’s Periodic Law was modified and the atomic number was adopted as the basis of Modern Periodic Table and the Modern Periodic Law can be stated as follows:
‘Properties of elements are a periodic function of their atomic number.’
Elements, when arranged in order of increasing atomic number Z, lead us to the classification known as the Modern Periodic Table. Prediction of properties of elements could be made with more precision when elements were arranged on the basis of increasing atomic number.
The Modern Periodic Table has 18 vertical columns known as ‘groups’ and 7 horizontal rows known as ‘periods’.
Activity
Look at group 1 of the Modern Periodic Table, and name the elements present in it. Write down the electronic configuration of the first three elements of group 1. What similarity do you find in their electronic configurations? You will find that all these elements contain the same number of valence electrons. Similarly, you will find that the elements present in any one group have the same number of valence electrons. For example, elements fluorine (F) and chlorine (Cl), belong to group 17, how many electrons do fluorine and chlorine have in their outermost shells? Hence, we can say that groups in the Periodic Table signify an identical outer shell electronic configuration. On the other hand, the number of shells increases as we go down the group.
Group's Patterns
Valance electron 👉 same
Shells 👉 increases
Activity
If you look at the long form of the Periodic Table, you will find that the elements Li, Be, B, C, N, O, F, and Ne are present in the second period. Write down their electronic configuration. Do these elements also contain the same number of valence electrons? Do they contain the same number of shells?
You will find that these elements do not have the same number of valence electrons, but they contain the same number of shells. You also observe that the number of valence shell electrons increases by one unit, as the atomic number increases by one unit on moving from left to right in a period.
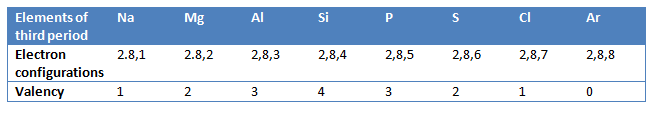
Or we can say that atoms of different elements with the same number of occupied shells are placed in the same period. Na, Mg, Al, Si, P, S, Cl, and Ar belong to the third period of the Modern Periodic Table since the electrons in the atoms of these elements are filled in K, L, and M shells.
Period's Patterns
Valance electron 👉 increases
Shells 👉 same
Characteristics of Periods
- Valency
- Size of atoms
- Metallic character
- Chemical reactivity
- Nature of oxides
1. Valency
The number of electrons lost or gained (or shared) by one atom of an element to achieve the nearest inert gas electron configuration, gives us the valency of the element. On moving from left to right in each short period, the valency of elements increases from 1 to 4 and then decreases to 0 (zero).
From the above table, we find that the valency of sodium (Na) is 1, magnesium (Mg) is 2, aluminium (Al) is 3, silicon (Si) is 4, phosphorous (P) is 3, sulphur (S) is 2, chlorine (Cl) is 1, and that of argon (Ar) is 0. Thus in the third period of the periodic table, the valency increases from 1 in sodium to 4 in silicon, and then decreases to zero in argon.
2. Size of the atoms (or atomic size)
The size of an atom is the distance between the centre of the nucleus and the outermost electron shell of an isolated atom. In other words, the size of an atom refers to the radius of the atom. Thus, the size of an atom is indicated by writing its radius called "atomic radius". The atomic radius is expressed in 'picometre' units whose symbol is 'pm'.
On moving from left to right in a period of the periodic table, the size of atoms decreases ( or atomic size decreases).
We will find that in the third period, sodium atom is the biggest size having an atomic radius of 186 pm whereas chlorine atom is the smallest having an atomic radius of 99 pm. As we move from left to right in a period, the atomic number of elements increases which means that the number of protons and electrons in the atoms increases (the extra electrons being added to the same shell). Due to the large positive charge on the nucleus, the electrons are pulled in more closely to the nucleus, and the size of the atom decreases.
3. Metallic character
On moving from left to right in a period, the metallic character of elements decreases (but the non-metallic character increases). On the left side in a period we have metals and on the right side, we have non-metals. Some elements in-between the metals and non-metals are known as metalloids.
In the third period of the periodic table, sodium, magnesium, and aluminium are metals. The properties of silicon are in between those of a metal and non-metal, therefore, silicon is a metalloid. The next elements, phosphorous, sulphur, and chlorine are non-metals. Thus, in the third period of the periodic table, sodium (Na) is the most metallic element whereas chlorine (Cl) is the most non-metallic element.
Metals lose electrons and form positive ions, so metals are called electropositive elements. On the other hand, non-metals accept electrons and form negative ions, so non-metals are called electronegative elements. On moving from left to right in a period, the electropositive character of elements decreases, but the electronegative character increases. As we move from left to right in a period of the periodic table, the nuclear charge (positive charge on the nucleus) increases due to a gradual increase in the number of protons. Due to the increase in the nuclear charge, the valence electrons are pulled in more strongly by the nucleus and it becomes more and more difficult for the atoms to lose electrons. Thus, on moving from left to right in a period, the tendency of atoms to lose electrons decreases. On the other hand, due to the increased nuclear charge, it becomes easier for the atoms to gain electrons. So, on moving from left to right in a period, the tendency of atoms to gain electrons increases.
4. Chemical reactivity
On moving from left to right in a period, the chemical reactivity of the elements first decreases and then increases.
In the first element of the third period, sodium, there is 1 valence electron which can lose easily to react with other substances, so it is very reactive. The second element magnesium has 2 valence electrons. It is not easy for an atom to lose 2 electrons, so magnesium is less reactive than sodium. Aluminium and silicon are still more unreactive (because they have 3 and 4 valence electrons respectively). Phosphorus has 5 valence electrons so it needs 3 more electrons to complete octet; sulphur has 6 valence electrons and needs 2 more electrons and chlorine has 7 valence electrons and needs 1 more electron to complete the 8 electron structures. Now, it is quite difficult for an atom to gain 3 electrons, it is easier to gain 2 electrons and it is very easy to gain 1 electron. So, the chemical reactivity increases from phosphorus to sulphur to chlorine.
5. Nature of oxides
On moving from left to right in a period, the basic nature of oxides decreases, and the acidic nature of oxides increases.
In the third period of the periodic table, sodium oxide is highly basic in nature and magnesium oxide is comparatively less basic. The aluminium and silicon oxides are amphoteric in nature. Phosphorus oxides are acidic, sulphur oxides are more acidic whereas chlorine oxides are highly acidic in nature.
Characteristics of groups
- Valence electrons
- Valency
- Size of atoms
- Metallic character
- Chemical reactivity
- Nature of oxides
1. Valence electrons
All the elements of a group of the periodic table have the same number of valence electrons. For example, all the elements of group 1 of the periodic table like lithium, sodium, potassium have 1 valence electron each in their atoms. The atoms of group 1 elements, lithium, sodium, and potassium, can lose their 1 valance electron easily to form positive ions like `Li^+`, `Na^+`, and `K^+` respectively, having 1 unit positive charge. So group 1 elements are monovalent (having valency of 1), and ionic in their chemical reaction.
All the elements of group 2 have 2 valence electrons each in their atoms. Group 2 elements are divalent (having valency of 2).
Group 13 → 3 valence electrons, Group 17 → 7 valence electrons, Group 18 → 8 valence electrons, due to this, the elements of group 18 are zerovalent (having zero valency) and unreactive.
2. Valency
Since the number of valence electrons in a group is the same, all the elements in a group have the same valency. For example, group 1 elements like lithium, sodium, potassium, etc all have 1 valence electron, so all elements of group 1 have the same valency of 1.
The main groups of the periodic table and the valencies of their elements are as follows:
- Valency of group 1 elements is 1
- Valency of group 2 elements is 2
- Valency of group 13 elements is 3
- Valency of group 14 elements is 4
- Valency of group 15 elements is 3
- Valency of group 16 elements is 2
- Valency of group 17 elements is 1
- Valency of group 18 elements is 0
3. Size of the atoms
Going down in a group of the periodic table, the size of atoms increases. For example, when we move down from top to bottom in group 1 of alkali metals, the size of the atoms increases gradually from lithium to francium.
Work in progress...
References
- Foundation Science Class 10 (Chemistry)
- NCERT Science Class 10
- S. Chand Chemistry Class 10
- https://en.wikipedia.org/wiki/Periodic_table





Nice
ReplyDeleteThank you
ReplyDeleteVery important and helpful notes 📝👍👍👍👍
ReplyDeleteThank you
ReplyDelete