Metals and Non-metals Class 10
Each of the 118 chemical elements discovered to date has a set of
characteristic properties. For the convenience of study, these elements have
been grouped on the basis of their common features into two broad classes,
metals and non-metals.
Characteristics properties of metals
Physical properties
1. Electronic configuration of metals
Metals atoms generally possesses 1, 2, or 3 electrons in their outermost
shells or valence shells. For example, the electronic configurations of
sodium, magnesium, and aluminium are given below.
Exceptions Hydrogen and helium
have in their outermost shells 1 and 2 electrons respectively, but they are
not metals. In these cases, the outermost shell is the first and only shell
(K shell).
2. Electropositive character Metals have high electropositivity, i.e., the metal atoms have a high
tendency to lose their valence electrons and become positive ions (cations).
By losing electrons a metal atom tends to acquire the stable configuration
of a nearest noble gas.
`Na \overset{-1e}{\rightarrow} Na^+` (Neon like configuration)
`Ca \overset{-2e}{\rightarrow} Ca^{2+}` (Argon like configuration)
`Al \overset{-3e}{\rightarrow} Al^{3+}` (Neon like configuration)
3. Malleability Metals are
malleable, i.e., they can be beaten into very thin sheets. For example, gold
and silver are among the most malleable metals.
4. Ductility Metals are
ductile, i.e., they can be drawn into wires. All the metals are not equally
ductile. Gold and silver are among the most ductile metals. It is estimated
that 100 mg of silver can be drawn into a wire of about 200 metres in
length. .
You will be surprised to know that a wire of about 2 km length can be drawn
from one gram of gold. Because of its ductile nature, copper is used in electrical
wires.
5. Thermal and electrical conductivity
All metals are good conductors of heat and electricity.
Silver is the best conductor of heat and electricity, while lead is the
poorest. The utensils we use in the kitchen are made of zinc, copper, and aluminium
because these metals are good conductors of heat.
All metals are good conductors of electricity because they contain free
electrons. These free electrons conduct electric currents.
Silver and copper are among the best conductors of electricity, followed
by gold, aluminium, and tungsten. Lead and mercury are comparatively poor conductors of heat.
The electrical conductivity of metals decreases with the rise in
temperature.
Exception Graphite is a good
conductor of electricity although it is a nonmetal.
6. Lustre Metals possess a
characteristic shining appearance called metallic lustre, and they can be
polished.
Exception Graphite and iodine
are lustrous but they are nonmetals.
7. Density Metals have high
densities, except sodium, potassium, etc., which have low densities. Because
of low densities, sodium, potassium, magnesium, and aluminium are called
light metals.
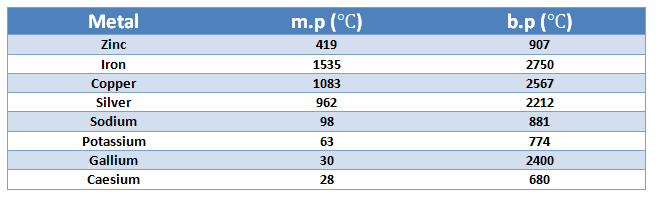
Densities
8. State Metals are generally
solids at room temperature,
except mercury, which is liquid at room temperature
9. Melting and boiling point Metals have high melting and boiling points.
But there are exceptions`-` sodium and potassium melt at low
temperatures. Gallium and caesium can melt if kept on the palm.
10. Sonorous The metals that produce a sound on striking a hard surface are said to be
sonorous.
11. Hardness Most metals are very
hard. However,
sodium and potassium are soft metals that can be cut with a knife.
12. Alloy formation Metals can
form homogenous mixtures in combination with other metals. Such a mixture is
called an alloy. For example, copper and zinc mix up to form
brass.
Exceptions
(i) All metals except mercury exist as solids at room temperature.
Metals have high melting points but gallium and caesium have very low
melting points. These two metals will melt if you keep them on your palm.
(ii) Iodine is a non-metal but it is lustrous.
(iii) Carbon is a non-metal that can exist in different forms. Each form
is called an allotrope. Diamond, an allotrope of carbon, is the hardest natural substance known
and has very high melting and boiling point. Graphite, another allotrope
of carbon, is a conductor of electricity.
(iv)
Alkali metals
(lithium, sodium, potassium) are so soft that they can be cut with a
knife. They have low densities and low melting points.
Characteristics properties of Non-metals
Physical properties
1. Non-metals are neither malleable nor ductile. Non-metals are
brittle (break easily)
Since non-metals are not malleable, they cannot be beaten with a hammer
to form thin shit. Again they cannot be stretched to form thin wires.
The property of being brittle (breaking easily) is called
brittleness.
Note that we can consider the brittleness of solid non-metals only. It
is not applicable to liquid or gaseous non-metals.
2. Non-metals do not conduct heat and electricity
Non-metals do not conduct heat and electricity because they have no
free electrons. There is however one exception,
carbon (in the form of graphite - allotropic form of carbon) is the
only non-metal which is a good conductor of electricity.
3. Non-metals are not lustrous (not shiny). They are dull.
Non-metals do not have a shining surface. For example, sulphur and
phosphorus are non-metals that have no lustre, they appear dull.
There is however an exception, iodine is a non-metal having a
lustrous appearance. It has a shining surface.
4. Non-metals are generally soft, except diamond (an allotropic form
of carbon) which is extremely hard.
5. Non-metals may be solid, liquid, or gases at room temperature
Non-metals can exist in all three physical states: solids, liquids, and
gaseous. For example, carbon, sulphur, and phosphorous are solids
non-metals; bromine is liquid non-metal; whereas hydrogen, oxygen,
nitrogen, and chlorine are gaseous non-metals.
6. Non-metals have comparatively low melting points and boiling
points
For example, the melting point of sulphur is 115°C.
The meting point of the diamond is however more than 3500°C, which is very high (exception).
7. Non-metals are low densities
The density of sulphur is `2g cm^{-3}`, which is quite low.
8. Non-metals are non-sonorous.
Chemical Properties of Metals
1. Reaction of Metals with Oxygen
Metal + Oxygen `rightarrow` Metal Oxide
When metals are burnt in the air, they react with oxygen to form metal
oxide. Metal oxides are basic in nature. Some of the metal oxides
react with water to form alkalies. Metal oxides being basic, turn red
litmus solution blue.
The vigour of reaction with oxygen depends on the chemical reactivity
of metals.
Some metals react with oxygen even at room temperature, some react on
heating, whereas still others react only on strong heating
Examples:
(a) Sodium metal reacts with oxygen at room temperature to
form a basic oxide called sodium oxide.
`4Na (s) + O_2 (g) rightarrow 2Na_2O (s)`
(b) Potassium metal reacts with oxygen at room temperature
to form potassium oxide.
`4K (s) + O_2 (g) rightarrow 2K_2O (s)`
Potassium and sodium are so reactive that they react vigorously with
oxygen. They catch fire and start burning when kept open in the air.
In fact, potassium and sodium metal are stored under kerosene oil to
prevent their reaction with oxygen, moisture, and carbon dioxide.
Therefore, `K` and `Na` are very reactive.
Lithium (Li) is very reactive, it is also stored under kerosene oil.
Most of the metal oxides are insoluble in water. But some of the metal
oxides dissolve in water to form alkalies.
Sodium and potassium oxide are two metal oxides which are soluble in
water.
Examples:
(i) Sodium oxide is a basic oxide which reacts with water to form an
alkali called sodium hydroxide.
`Na_2O (s) + H_2O (l) rightarrow 2NaOH (aq)`
(ii) `K_2O (s) + H_2O (l) rightarrow 2KOH (aq)`
(c) Magnesium metal does not react with oxygen at room temperature.
But on heating, magnesium metal burns in the air giving intense heat and
light to form a basic oxide called magnesium oxide.
`2Mg (s) + O_2 (g) rightarrow 2MgO (s)`
Since heat is required for the reaction of magnesium with oxygen, it
means magnesium is less reactive than sodium (or potassium).
Magnesium oxide dissolves in water partially to form a magnesium hydroxide
solution.
`MgO (s) + H_2O rightarrow Mg(OH)_2 (aq)`
(d) Aluminium metal burns in air, on heating to form aluminium
oxide
`4Al (s) + 3O_2 (g) rightarrow 2Al_2O_3 (s)`
Since the reaction of aluminium with oxygen takes place less readily
than magnesium, so aluminium is less reactive than magnesium.
Amphoteric oxide
Though most of the metal oxides are basic in nature but some of the metal
oxides show basic as well as acidic nature.
Those metal oxides which show basic, as well as acidic behaviour, are
known as amphoteric oxides.
Aluminium and zinc metal form amphoteric oxides. Amphoteric oxides react
with both acids as well as bases to form salts and water.
Examples:
`Al_2O_3 (s) + 6HCl (aq) rightarrow 2AlCl_3 (aq) + 3H_2O (l)`
Aluminium oxide reacts with sodium hydroxide to form sodium aluminate
(salt).
`Al_2O_3 (s) + 2NaOH (aq) rightarrow 2NaAlO_2 (aq) + H_2O (l)`
(e) Zinc metal burns in the air only on strong heating to form zinc oxide.
`2Zn (s) + O_2 (g) rightarrow 2ZnO (s)`
Since the reaction of zinc with oxygen takes place less than aluminium, so zinc is less reactive than aluminium.
Zinc oxide is an amphoteric oxide
Examples:
Zinc oxide reacts with hydrochloric acid to form zinc chloride (salt) and water.
`ZnO (s)+ 2HCl (aq) rightarrow ZnCl_2 (aq) + H_2O (l)`
Zinc oxide reacts with sodium hydroxide to form sodium zincate (salt) and water.
`ZnO (s) + 2NaOH (aq) rightarrow Na_2ZnO_2 (aq) + H_2O (l)`
(f) Iron metal does not burn in air even on strong heating. Iron reacts with the oxygen of air on heating to form iron (II, III) oxide.
`2Fe (s) + O_2 (g) rightarrow Fe_3O_4 (s)`
Thus, the reaction of iron with oxygen takes less readily than that of zinc, so iron is less reactive than zinc.
(g) Copper metal reacts with oxygen on prolonged heating to form copper (II) oxide.
`2Cu (s) + O_2 (g) rightarrow 2CuO (s)`
Since the reaction of copper with oxygen takes place even less readily than that of iron, so copper is less reactive than iron.
(h) Silver and gold metals do not react with oxygen even at high temperatures.
2. Reaction of Metals with Water
Metal + Water `rightarrow` Metal hydroxide `+` Hydrogen
Metal + Steam `rightarrow` Metal oxide `+` Hydrogen
Metals react with water to form a metal hydroxide (or metal oxide) and hydrogen gas. But all metals do not react with water.
➤ Metals like potassium and sodium react violently with cold water. In the case of sodium and potassium, the reaction is so violent and exothermic that the evolved hydrogen immediately catches fire.
`2K (s) + 2H_2O (l) rightarrow 2KOH (aq) + H_2 (g)` + heat energy
`2Na (s) + 2H_2O (l) rightarrow 2NaOH (aq) + H_2 (g)` + heat energy
➤ The reaction of calcium with water is less violent. The heat evolved is not sufficient for the hydrogen to catch fire. Calcium starts floating because the bubbles of hydrogen gas formed stick to the surface of the metal.
`Ca (s) + 2H_2O (l) rightarrow Ca(OH)_2 (aq) + H_2 (g)`
➤ Magnesium does not react with cold water. It reacts with hot water to form magnesium hydroxide and hydrogen. It also starts floating due to the bubbles of hydrogen gas sticking to its surface.
`Mg (s) + 2H_2O (l) rightarrow Mg(OH)_2 (aq) + H_2 (g)`
➤ Metals like aluminium, iron and zinc do not react either with cold or hot water. But they react with steam to form metal oxide and hydrogen.
`2Al (s) + 3H_2O (g) rightarrow Al_2O_3 (s) + 3H_2 (g)`
`2Fe (s) + 4H_2O (g) rightarrow Fe_3O_4 (s) + 4H_2 (g)`
➤ Metals such as lead, copper, silver and gold do not react with water at all.
3. Reaction of Metals with Dilute acids
Metals usually displace hydrogen from dilute acids. Only less reactive metals like copper, silver, and gold do not displace hydrogen from dilute acids.
Metal + Dilute Acid `rightarrow` Metal Salts + Hydrogen
(i) Sodium metal reacts violently with dilute hydrochloric acid to form sodium chloride and hydrogen gas.
`2Na (s) + 2HCl (aq) rightarrow 2NaCl (aq) + H_2 (g)`
(ii) Magnesium reacts quite rapidly with dilute hydrochloric acid forming magnesium chloride and hydrogen gas.
`Mg (s) + 2HCl (aq) rightarrow MgCl_2(aq) + H_2 (g)`
The reaction of magnesium with dilute hydrochloric acid is less vigorous than sodium, so magnesium is less reactive than sodium.
(iii) Aluminium metal reacts rapidly with dilute hydrochloric acid to form aluminium chloride and hydrogen gas.
`2Al (s) + 6HCl (aq) rightarrow 2AlCl_3 (aq) + 2H_2 (g)`
The reaction of aluminium with dilute hydrochloride acid is less rapid than that of magnesium, so aluminium is less reactive than magnesium.
(iv) Zinc metal reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas (but the reaction is less rapid than that of aluminum.)
`Zn (s) + 2HCl (aq) rightarrow ZnCl_2 (aq) + H_2 (g)`
(v) Iron reacts slowly with dilute hydrochloric acid to give iron (II) chloride and hydrogen gas
`Fe (s) + 2HCl (aq) rightarrow FeCl_2 (aq) + H_2`
This shows that iron is less reactive than zinc.
(vi) Copper does not react with dilute hydrochloric acid (or sulphuric acid).
`Cu (s) + HCl (aq) rightarrow` No reaction
(vii) Silver and gold do not react with dilute acids.
➤ All those metals which are more reactive than hydrogen, displace hydrogen from dilute acids to produce hydrogen gas. The metals like copper and silver which are less reactive than hydrogen, do not displace hydrogen from dilute acids.
➤ Hydrogen gas is not evolved when a metal reacts with nitric acid. It is because `HNO_3` is a strong oxidising agent. It oxidises the `H_2` produced to water and itself gets reduced to any of the nitrogen oxides `(N_2O, NO, NO_2)`. But magnesium `(Mg)` and manganese `(Mn)` react with very dilute `HNO_3` to evolve `H_2` gas.
`Mg (s) + 2HNO_3 (aq) rightarrow Mg(NO_3)_2 (aq) + H_2 (g)`
`Mn (s) + 2HNO_3 (aq) rightarrow Mn(NO_3)_2 (aq) + H_2 (g)`
The Reactivity Series of Metals (or Activity Series of Metals)
➤ The arrangement of metals in a vertical column in the order of decreasing reactivities is called reactivity series of metals (or activity series of metals).
Though hydrogen is not a metal, even then it has been placed in the reactivity series. This is because hydrogen also loses electrons and forms positive ions `H^+`.
Reaction of metals with salt solutions
➤ Reactive metals can displace less reactive metals from their compounds in solution or molten form.
Reaction of metals with salt solutions
`Fe (s) + CuSO_4 (aq) rightarrow FeSO_4 (aq) + Cu (s)`
[Iron + Copper (II) sulphate (Blue colour) `rightarrow` Iron (II) sulphate or Ferrous sulphate (Greenish colour) + Copper]
How do metals and non-metals react?
We learned that noble gases, which have a completely filled valence
shell, show little chemical activity. We, therefore, explain the
reactivity of elements as a tendency to attain a completely filled
valence shell.
Let us have a look at the electronic configuration of noble gases and
some metals and non-metals. We can see from the Table that a sodium
atom has one electron in its outermost shell.
Electronic configurations of some elements
If it loses the electron from its M shell then its L shell now
becomes the outermost shell and that has a stable octet. The nucleus
of this atom still has 11 protons but the number of electrons has
become 10, so there is a net positive charge giving us a sodium
cation `Na^{+}`. On the other hand, chlorine has seven electrons in
its outermost shell and it requires one more electron to complete
its octet. If sodium and chlorine were to react, the electron lost
by sodium could be taken up by chlorine. After gaining an electron,
the chlorine atom gets a unit negative charge, because its nucleus
has 17 protons and there are 18 electrons in its K, L, and M shells.
This gives us a chloride anion `Cl^{-}`. So both these
elements can have a give-and-take relation between them as
follows
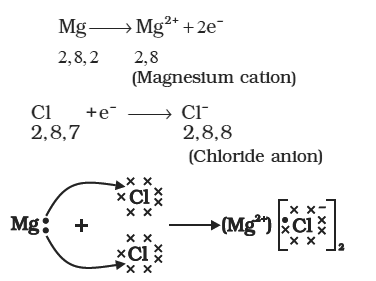
Formation of Sodium chloride
Sodium and chloride ions, being oppositely charged, attract each
other and are held by strong electrostatic forces of attraction to
exist as sodium chloride `(NaCl)`. It should be noted that sodium
chloride does not exist as molecules but aggregates of oppositely
charged ions.
Let us see the formation of one more ionic compound, magnesium
chloride
Formation of Magnesium chloride
The compounds formed in this manner by the transfer of
electrons from a metal to a non-metal are known as ionic
compounds or electrovalent compounds.
Properties of Ionic Compounds
Melting and boiling points of some ionic compounds
(i) Physical nature: Ionic compounds are solids and are somewhat hard because of the strong force of attraction between the positive and negative ions. These compounds are generally brittle and break into pieces when pressure is applied.
(ii) Melting and Boiling points: Ionic compounds have high melting and boiling points. This is because a considerable amount of energy is required to break the strong inter-ionic attraction.
(iii) Solubility: Electrovalent compounds are generally soluble in water and insoluble in solvents such as kerosene, petrol, etc.
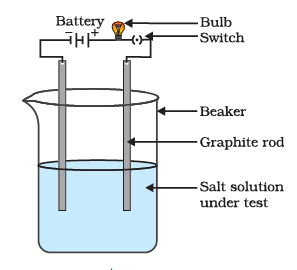
(iv) Conduction of Electricity: The conduction of electricity through a solution involves the movement of charged particles. A solution of an ionic compound in water contains ions, which move to the opposite electrodes when electricity is passed through the solution. Ionic compounds in the solid state do not conduct electricity because the movement of ions in the solid is not possible due to their rigid structure. But ionic compounds conduct electricity in the molten state. This is possible in the molten state since the electrostatic forces of attraction between the oppositely charged ions are overcome due to the heat. Thus, the ions move freely and conduct electricity.
Testing the conductivity of a salt solution
Extracting Metals in the Middle of the Activity Series
👉The metals in the middle of the activity series such as iron, zinc, lead,
copper, are moderately reactive.
👉These are usually present as sulphides
or carbonates in nature.
👉 It is easier to obtain a metal from its oxide (by reduction), as
compared to its sulphides and carbonates. Therefore, prior to reduction,
the metal sulphides and carbonates must be converted into metal
oxides.
👉The sulphide ores are converted into oxides by heating strongly
in the presence of excess air. This process is known as roasting.
👉 The
carbonate ores are changed into oxides by heating strongly in limited
air or the absence of air. This process is known as calcination.
Roasting
Calcination
👉The metal oxides are then reduced to the corresponding metals by using suitable reducing agents such as carbon (coke).
👉 Besides using carbon (coke) to reduce metal oxides to metals, the highly reactive
metals such as sodium, calcium, aluminium, etc., are used as reducing agents because they can displace metals of lower reactivity from their
compounds. For example, when manganese dioxide is heated with
aluminium powder, the following reaction takes place.
👉These displacement reactions are highly exothermic. The
amount of heat evolved is so large that the metals are produced
in the molten state.
👉The reaction of iron(III) oxide `(Fe_2O_3)` with aluminium is used to join railway tracks or cracked
machine parts. This reaction is known as the thermit reaction.
Work in progress
Summary
Reaction of Metals with Water
Metal + Water `rightarrow` Metal hydroxide `+` Hydrogen
Metal + Steam `rightarrow` Metal oxide `+` Hydrogen
👉Reaction with cold water
`2K (s) + 2H_2O (l) rightarrow 2KOH (aq) + H_2 (g)` + heat energy
`2Na (s) + 2H_2O (l) rightarrow 2NaOH (aq) + H_2 (g)` + heat energy
Catches fire 👆
`Ca (s) + 2H_2O (l) rightarrow Ca(OH)_2 (aq) + H_2 (g)`
Float 👆
👉Reaction with hot water
`Mg (s) + 2H_2O (l) rightarrow Mg(OH)_2 (aq) + H_2 (g)`
Float 👆
👉Reaction with steam
`2Al (s) + 3H_2O (g) rightarrow Al_2O_3 (s) + 3H_2 (g)`
`2Fe (s) + 4H_2O (g) rightarrow Fe_3O_4 (s) + 4H_2 (g)`
👉Metals such as lead, copper, silver and gold do not react with water at all.
Corrosion
Corrosion is the gradual deterioration of a material, usually metal, caused by a chemical reaction with its environment, such as rust forming on iron when it reacts with oxygen and moisture.
👉 Silver articles become black after some time when exposed to air.
This is because it reacts with sulphur in the air to form a coating
of silver sulphide.
👉 Copper reacts with moist carbon dioxide in the air and slowly loses
its shiny brown surface and gains a green coat. This green
substance is basic copper carbonate.
👉Iron when exposed to moist air for a long time acquires a coating
of a brown flaky substance called rust `(Fe_2O_3.xH_20)` hydrated iron (iii) oxide.
Let us find out the conditions under which iron rusts.
🔍Activity:
1. Take three test tubes and place clean iron nails
in each of them.
2. Label these test tubes A, B and C. Pour some
water in test tube A and cork it.
3. Pour boiled distilled water in test tube B, add
about 1 mL of oil and cork it. The oil will float on
water and prevent the air from dissolving in the
water.
4. Put some anhydrous calcium chloride in
test tube C and cork it. Anhydrous calcium
chloride will absorb the moisture, if any, from
the air. Leave these test tubes for a few days and
then observe (Fig. 3.13).
👉 Iron nails rust in test tube A,
but they do not rust in test tubes B and C. In the test
tube A, the nails are exposed to both air and water.
👉 In
the test tube B, the nails are exposed to only water, and
the nails in test tube C are exposed to dry air.
Quick glance
●Elements can be classified as metals and non-metals.
● Metals are lustrous, malleable, ductile, and are good conductors of heat and electricity. They are solids at room temperature, except mercury which is a liquid.
● Metals can form positive ions by losing electrons to non-metals.
●Metals combine with oxygen to form basic oxides. Aluminium oxide and zinc oxide show the properties of both basic as well as acidic oxides. These oxides are known as amphoteric oxides.
● Different metals have different reactivities with water and dilute acids.
● A list of common metals arranged in order of their decreasing reactivity is known as an activity series.
● Metals above hydrogen in the Activity series can displace hydrogen from dilute acids.
● A more reactive metal displaces a less reactive metal from its salt solution.
● Metals occur in nature as free elements or in the form of their compounds.
● The extraction of metals from their ores and then refining them for use is known as metallurgy.
● An alloy is a homogeneous mixture of two or more metals, or a metal and a non-metal.
● The surface of some metals, such as iron, is corroded when they are exposed to moist air for a long period of time. This phenomenon is known as corrosion.
● Non-metals have properties opposite to that of metals. They are neither malleable nor ductile. They are bad conductors of heat and electricity, except for graphite, which conducts electricity.
● Non-metals form negatively charged ions by gaining electrons when reacting with metals.
● Non-metals form oxides which are either acidic or neutral.
● Non-metals do not displace hydrogen from dilute acids. They react with hydrogen to form hydrides.
References
- NCERT Science Class X
- Foundation Science Chemistry Class X
- S. Chand Chemistry Class X











Education is my life
ReplyDeleteGood note jay sir
ReplyDeleteImportant notes sir 👍
ReplyDeleteThanks
DeleteNice notes sir
ReplyDeleteThank you
DeleteNice notes 👍
ReplyDelete👍👍👍
ReplyDeleteThank you
DeleteVery understanding notice sir 👍👍👍👍🙏🙏
ReplyDeleteThank you
DeleteNice and knowledge full notes
ReplyDelete🤗🤗🤗🙏🙏
Very nice notes sir
ReplyDelete