📝 Metals and Non-metals Questions
Multiple Choice Questions
1. Which of the following property is generally not shown by metals?
(a) Electrical conduction(b) Sonorous in nature
(c) Dullness
(d) Ductility
Ans: (c)
2. The ability of metals to be drawn into the thin wire is known as
(a) ductility
(b) malleability
(c) sonorousity
(d) conductivity
Ans: (a)
3. Aluminium is used for making cooking utensils. Which of the following properties of aluminium are responsible for the same?
(i) Good thermal conductivity (ii) Good electrical conductivity (iii) Ductility (iv) High melting point
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv)
Ans: (d)
4. Which of the following metals exist in their native state in nature?
(i) Cu (ii) Au (iii) Zn (iv) Ag
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Ans: (c)
5. Stainless steel is a very useful material for our life. In stainless steel, iron is mixed with
(a) Ni and Cr
(b) Cu and Cr
(c) Ni and Cu
(d) Cu and Au
Ans: (a)
6. If copper is kept open in the air, it slowly loses its shining brown surface and gains a green coating. It is due to the formation of
(a) `CuSO_4`
(b) `CuCO_3`
(c) `Cu(NO_3)_2`
(d) `CuO`
Ans: (b)
7. Generally, metals are solid in nature. Which one of the following metals is found in the liquid state at room temperature?
(a) Na
(b) Fe
(c) Cr
(d) Hg
Ans: (d)
8. Generally, non-metals are not lustrous. Which of the following nonmetal is lustrous?
(a) Sulphur
(b) Oxygen
(c) Nitrogen
(d) Iodine
Ans: (d)
9. An alloy is
(a) an element
(b) a compound
(c) a homogeneous mixture
(d) a heterogeneous mixture
Ans: (c)
10. An element A is soft and can be cut with a knife. This is very reactive to air and cannot be kept open in air. It reacts vigorously with water. Identify the element from the following
(a) Mg
(b) Na
(c) P
(d) Ca
Ans: (b)
11. Generally, non-metals are not conductors of electricity. Which of the following is a good conductor of electricity?
(a) Diamond
(b) Graphite
(c) Sulphur
(d) Fullerene
Ans: (b)
12. Alloys are homogeneous mixtures of a metal with a metal or nonmetal. Which among the following alloys contain non-metal as one of its constituents?
(a) Brass
(b) Bronze
(c) Amalgam
(d) Steel
Ans: (d)
13. Electrical wires have a coating of insulating material. The material, generally used is
(a) Sulphur
(b) Graphite
(c) PVC
(d) All can be used
Ans: (c)
14. Although metals form basic oxides, which of the following metals form an amphoteric oxide?
(a) Na
(b) Ca
(c) Al
(d) Cu
Ans: (c)
15. Which of the following non-metals is a liquid?
(a) Carbon
(b) Bromine
(c) Phosphorus
(d) Sulphur
Ans: (b)
16. Which of the following oxide(s) of iron would be obtained on the prolonged reaction of iron with steam?
(a) `FeO`
(b) `Fe_2O_3`
(c) `Fe_3O_4`
(d) `Fe_2O_3` and `Fe_3O_4`
Ans: (c)
17. What happens when calcium is treated with water?
(i) It does not react with water
(ii) It reacts violently with water
(iii) It reacts less violently with water
(iv) Bubbles of hydrogen gas formed stick to the surface of calcium
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
Ans: (d)
18. Silver articles become black on prolonged exposure to air. This is due to the formation of
(a) `Ag_3N`
(b) `Ag_2O`
(c) `Ag_2S`
(d) `Ag_2S` and `Ag_3N`
Ans: (c)
19. Which among the following statements is incorrect for magnesium metal?
(a) It burns in oxygen with a dazzling white flame
(b) It reacts with cold water to form magnesium oxide and evolves hydrogen gas
(c) It reacts with hot water to form magnesium hydroxide and evolves hydrogen gas
(d) It reacts with steam to form magnesium hydroxide and evolves hydrogen gas
Ans: (b)
20. Which of the following is a characteristic of metals?
(a) They have one to three valence electrons
(b) They have 4 to 8 valence electrons
(c) They are brittle
(d) They are capable to form anions easily
Ans: (a)
21. Which of the following metal has the highest melting point?
(a) Copper
(b) Silver
(c) Sodium
(d) Tungsten
Ans: (d)
22. Which of the following reaction shows that the given oxide is amphoteric in nature?
(a) `2Zn + O_2 overset{triangle}rightarrow 2ZnO`
(b) `ZnO + H_2SO_4 rightarrow ZnSO_4 + H_2O`
(c) `ZnO + 2NaOH rightarrow Na_2ZnO_2 + H_2O`
(d) (b) and (c) together
Ans: (d)
(b). i and iv
(c). ii and iii
(d). ii and iv
Ans: (d)
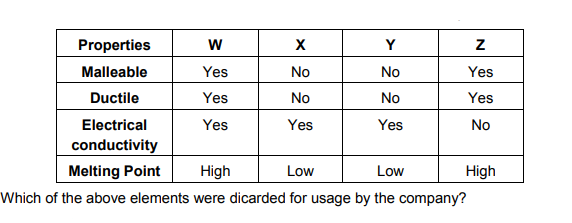
24. A cable manufacturing unit tested a few elements on the basis of their physical properties.
(a). W, X, Y (b). X, Y, Z
(c). W, X, Z
(d). W, X, Z
Ans: (b)
25. Which of the following pairs will give displacement reactions?
(a) `NaCl` solution and copper metal
(b) `MgCl_2` solution and aluminium metal
(c) `FeSO_4` solution and silver metal
(d) `AgNO_3` solution and copper metal.
Ans: (d)
26. Generally metals react with acids to give salt and hydrogen gas. Which of the following acids does not give hydrogen gas on reacting with metals (except Mn and Mg)?
(a) `H_2SO_4`
(b) `HCl`
(c) `HNO_3`
(d) All of these
Ans: (c)
27. Which of the following are not ionic compounds?
(i)` KCl`
(ii) `HCl`
(iii) CC`l_4`
(iv) `NaCl`
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iii)
Ans: (b)
28. Which one of the following properties is not generally exhibited by ionic compounds?
(a) Solubility in water
(b) Electrical conductivity in solid state
(c) High melting and boiling points
(d) Electrical conductivity in molten state
Ans: (b)
29. Metals are refined by using different methods. Which of the following metals are refined by electrolytic refining?
(i) Au
(ii) Cu
(iii) Na
(iv) K
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (iii) and (iv)
Ans: (a)
30. Galvanisation is a method of protecting iron from rusting by coating it with a thin layer of
(a) Gallium
(b) Aluminium
(c) Zinc
(d) Silver
Ans: (c)
31. Which of the following metals are obtained by electrolysis of their chlorides in a molten state?
(i) Na
(ii) Ca
(iii) Fe
(iv) Cu
(a) (i) and (iv)
(b) (iii) and (iv)
(c) (i) and (iii)
(d) (i) and (ii)
Ans: (d)
32. 2ml each of concentrated `HCl, HNO_3` and a mixture of concentrated HCl and concentrated `HNO_3` in the ratio of 3 : 1 were taken in test tubes labelled as A, B and C. A small piece of metal was put in each test tube. No change occurred in test tubes A and B but the metal got dissolved in test tube C respectively. The metal could be
(a) `Al`
(b) `Au`
(c) `Cu`
(d) `Pt`
Ans: (b)
33. An electrolytic cell consists of
(i) positively charged cathode
(ii) negatively charged anode
(iii) positively charged anode
(iv) negatively charged cathode
(a) (i) and (ii)
(b) (iii) and (iv)
(c) (i) and (iii)
(d) (ii) ad (iv)
Ans: (b)
34. During electrolytic refining of zinc, it gets
(a) deposited on cathode
(b) deposited on anode
(c) deposited on cathode as well as anode
(d) remains in the solution
Ans: (a)
35. Which of the following alloys contain mercury as one of its constituents?
(a) Stainless steel
(b) Alnico
(c) Solder
(d) Zinc amalgam
Ans: (d)
36. Reaction between X and Y, forms compound Z. X loses electron and Y gains electron. Which of the following properties is not shown by Z?
(a) Has high melting point
(b) Has low melting point
(c) Conducts electricity in molten state
(d) Occurs as solid
Ans: (b)
37. The electronic configurations of three elements X, Y and Z are X — 2, 8; Y — 2, 8, 7 and Z — 2, 8, 2. Which of the following is correct?
(a) X is a metal
(b) Y is a metal
(c) Z is a non-metal
(d) Y is a non-metal and Z is a metal
Ans: (d)
38. Which of the following can undergo a chemical reaction?
(a) `MgSO_4 + Fe`
(b) `ZnSO_4 + Fe`
(c) `MgSO_4 + Pb`
(d) `CuSO_4 + Fe`
Ans: (d)
39. Which one of the following figures correctly describes the process of electrolytic refining?
Ans: (c)
40. The composition of aqua-regia is
(a) `Dil.HCl : Conc. HNO_3 = 3:1`
(b) `Conc.HCl : Dil. HNO_3 = 3:1`
(c) `Conc.HCl : Conc.HNO_3 = 3:1`
(d) `Dil.HCl : Dil.HNO_3 = 3:1`
Ans: (c)
★★★★★★
Assertion & Reason
(a) If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
(b) If both Assertion and Reason are true and Reason is not the correct explanation of Assertion.
(c) If Assertion is true but Reason is false.
(d) If Assertion is false but Reason is true.
1. Assertion: When zinc is added to a solution of iron (II) sulphate, no change is observed.
Reason: Zinc is more reactive than iron.
Ans: (d) If Assertion is false but Reason is true.
2. Assertion: Platinum, gold, and silver are used to make jewellery.
Reason: Plantium, gold, and silver are the least reactive metals.
Ans: (a) If both Assertion and Reason are true and Reason is the correct explanation of Assertion
★★★★★★
Case Studies
1. A student performs some activities on two substances and records the observations in a table as shown.
Which option classifies the substances into metals and non-metals?
(a) both the substances are metals
(b) both the substances are non-metals
(c) substance M is metal while substance N is non-metal
(d) substance M is non-metal while substance N is metal
Ans: (c)
2. Which option classifies the substances based on their physical properties?




Very hard and tuesed questions sir👍👍👌👌💥💥
ReplyDeleteThank you
DeleteVery important and help full question sir ☺
ReplyDeleteThank you
Delete👌👌👌
ReplyDeleteThank you
DeleteVery good questions ✌👍✌👍👍
ReplyDeleteThank you
DeleteSuper sa be uperwal questions ha sir
ReplyDeleteThank You
DeleteVery important questions 👍
ReplyDeleteThank you
Delete"A GOOD EDUCATION 📚 CAN CHANGE ANYONE...........
ReplyDeleteA GOOD TEACHER 👨🏫 👩🏫 CAN CHANGE EVERYTHING...........