SOME BASIC CONCEPTS OF CHEMISTRY
{getToc} $title={Table of Contents}
Science can be viewed as a continuing human effort to
systematise knowledge for describing and understanding
nature. For the sake of convenience,
science is sub-divided into various disciplines: chemistry,
physics, biology, etc. The branch of science that
studies the preparation, properties, structure and reactions
of material substances is called chemistry.
Chemistry deals with the composition,
structure, properties and interactions of matter
and is of much use to human beings in daily
life. These aspects can be best described and
understood in terms of basic constituents of
matter are atoms and molecules. That
is why chemistry is also called the science of
atoms and molecules.
Matter
Anything which has
mass and occupies space is called matter.
Everything around us, for example, book, pen,
pencil, water, air, all living beings, etc., are
composed of matter. You know that they have
mass and they occupy space.
States of Matter
Particles are held very close to each other
in solids in an orderly fashion and there is not
much freedom of movement. In liquids, the
particles are close to each other but they can
move around. However, in gases, the particles
are far apart as compared to those present in
solid or liquid states and their movement is
easy and fast.
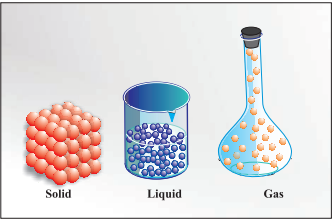 |
Arrangement of particles in solid, liquid and gaseous state
(Image credit: NCERT) |
Because of such arrangement
of particles, different states of matter exhibit
the following characteristics
(i) Solids have definite volume and definite
shape.
(ii) Liquids have definite volume but do not
have a definite shape. They take the shape
of the container in which they are placed.
(iii) Gases have neither definite volume nor
definite shape. They completely occupy the
space in the container in which they are placed.
These three states of matter are
interconvertible by changing the conditions of
temperature and pressure
Classification of Matter
 |
| Classification of matter (Image credit: NCERT) |
At the macroscopic or bulk level, matter can be
classified as mixture or pure substance. These
can be further sub-divided
Mixture
A mixture contains particles of two or more
pure substances which may be present in it in
any ratio. Hence, their composition is variable.
Pure substances forming mixture are called its
components. Many of the substances present
around you are mixtures. For example, sugar
solution in water, air, tea, etc., are all mixtures.
Mixture (sub-divided)
- Homogeneous
- Heterogeneous.
Homogeneous
In a homogeneous mixture,
the components completely mix with each other.
This means particles of components of the
mixture are uniformly distributed throughout the bulk of the mixture and its composition is
uniform throughout.
Sugar solution and air
are examples of homogeneous mixtures.
Heterogeneous
The composition is not uniform
throughout and sometimes different
components are visible. For example, mixtures
of salt and sugar, grains and pulses along with
some dirt (often stone pieces), are
heterogeneous mixtures.
👉 The components of a
mixture can be separated by using physical
methods, such as simple
hand-picking, filtration, crystallisation,
distillation, etc
Pure substances
Constituent particles
of pure substances have fixed composition.
Copper, silver, gold, water and glucose are
some examples of pure substances. Glucose
contains carbon, hydrogen and oxygen in a
fixed ratio and its particles are of same
composition.
👉 Its constituents—carbon, hydrogen, and
oxygen—cannot be separated by simple
physical methods.
Pure substances (Sub-divided)
Elements
Particles of an element consist of only one
type of atoms. These particles may exist as
atoms or molecules.
Some elements, such as sodium or
copper, contain atoms as their constituent
particles, whereas, in some others, the
constituent particles are molecules which
are formed by two or more atoms. For
example, hydrogen, nitrogen and oxygen
gases consist of molecules, in which two
atoms combine to give their respective
molecules.
 |
| A representation of atoms and molecules (Image credit: NCERT) |
Compounds
When two or more atoms of different
elements combine together in a definite ratio,
the molecule of a compound is obtained.
LAWS OF CHEMICAL
COMBINATIONS
The combination of elements
to form compounds is
governed by the following five
basic laws
- Law of Conservation of Mass
- Law of Definite Proportions
- Law of Multiple Proportions
- Gay Lussac’s Law of Gaseous
Volumes
- Avogadro’s Law
1. Law of Conservation of Mass
In all physical and
chemical changes, there is no net change in
mass during the process. Hence, he reached the conclusion that matter can neither be
created nor destroyed.
In fact, this was the result of the exact
measurement of masses of reactants and
products, and carefully planned experiments
performed by Lavoisier
2. Law of Definite Proportions
A given
compound always contains
exactly the same proportion
of elements by weight.
3. Law of Multiple Proportions
If two elements can
combine to form more than one compound, the
masses of one element that combine with a
fixed mass of the other element, are in the
ratio of small whole numbers.
For example, hydrogen combines with
oxygen to form two compounds, namely, water
and hydrogen peroxide.
Here, the masses of oxygen (i.e., 16 g and 32 g),
which combine with a fixed mass of hydrogen
(2g) bear a simple ratio, i.e., 16:32 or 1: 2
4. Gay Lussac’s Law of Gaseous
Volumes
When gases combine or are produced in a chemical
reaction they do so in a
simple ratio by volume,
provided all gases are at
the same temperature and
pressure.
Thus, 100 mL of hydrogen
combine with 50 mL of oxygen
to give 100 mL of water
vapour.
Thus, the volumes of hydrogen and oxygen
which combine (i.e., 100 mL and
50 mL) bear a simple ratio of 2:1.
5. Avogadro’s Law
Equal
volumes of all gases at the same temperature
and pressure should contain equal number
of molecules.
 |
Two volumes of hydrogen react with one volume of oxygen to give two volumes of water vapour
(Image credit: NCERT)) |
Atomic Masses
In 1961 for a universally
accepted atomic mass unit, carbon-12 isotope
was chosen as the standard reference for
measuring atomic masses. One atomic mass
unit is a mass unit equal to exactly one-twelfth
(1/12th) the mass of one atom of carbon-12.
The relative atomic masses of all elements
have been found with respect to an atom of
carbon-12.
One atomic
mass unit is defined as a mass exactly equal to one-twelfth of the mass of one carbon - 12
atom.
1 amu `= 1.66056×10^{–24}` g
Mass of an atom of hydrogen `= 1.6736×10^{–24}` g
At present,
‘amu’ has been replaced by
‘u’,
which is known as
unified mass.
Average Atomic Mass
Many naturally occurring elements exist as
more than one isotope. For example, carbon has the
following three isotopes with relative
abundances and masses as shown against each of them.
 |
| Image credit: NCERT |
From the above data, the average atomic
mass of carbon will come out to be:
`(0.98892) (12 u) + (0.01108) (13.00335 u) + (2 × 10^{–12}) (14.00317 u) = 12.011 u`
Molecular Mass
Molecular mass is the sum of the atomic masses
of the elements present in a molecule. It is
obtained by multiplying the atomic mass of
each element by the number of its atoms and
adding them together.
Molecular mass of methane,
`CH_4 = (12.011 u) + 4 (1.008 u)
= 16.043 u`
Formula Mass
Some substances, such as sodium chloride,
do not contain discrete molecules as their
constituent units. In such compounds, positive
(sodium ion) and negative (chloride ion) entities
are arranged in a three-dimensional structure,
as shown in Fig.
 |
| Image credit: NCERT |
The formula, such as `NaCl`, is used to
calculate the formula mass instead of molecular
mass as in the solid state sodium chloride does
not exist as a single entity.
Thus, the formula mass of sodium chloride is the atomic mass of sodium `+` atomic mass of chlorine
`= 23.0 u + 35.5 u = 58.5 u`
MOLE CONCEPT AND MOLAR
MASSES
The number of particles (atoms, molecules
or ions) present in 1 mole of any substance is
fixed, with a value of `6.022 × 10^{23}`. This is an
experimentally obtained value. This number
is called the Avogadro Constant or Avogadro
Number (represented by `N_A`), named in honour
of the Italian scientist, Amedeo Avogadro.
1 mole ` = 6.022 × 10^{23}` in number,
as, 1 dozen = 12 nos.
1 gross = 144 nos.
The mass of 1 mole of a substance is equal
to its relative atomic or molecular mass in
grams. We have to take the same numerical
value but change the units from ‘u’ to ‘g’. Molar
mass of atoms is also known as gram atomic
mass.
For example, atomic mass of
hydrogen `=1u`. So, gram atomic mass of
hydrogen `= 1 g`. `1 u` hydrogen has only 1 atom of hydrogen
1 g hydrogen has 1 mole atoms, that is, `6.022 × 10^{23}` atoms of hydrogen.
18 u water has only 1 molecule of water,
18 g water has 1 mole molecules of water, that
is, `6.022 × 10^{23}` molecules of water.
1 mole `= 6.022 × 10^{23}`
`=` Relative mass in grams.
The word “mole” was introduced around
1896 by Wilhelm Ostwald who derived the
term from the Latin word moles meaning a
‘heap’ or ‘pile’. A substance may be considered
as a heap of atoms or molecules.
Calculations
`n =` No. of moles
`m =` given mass
`M =` Molar mass
`n = m/M`
`N =` Given no of particles
`N_A =` Avogadro number
`n = N/N_A`
EMPIRICAL FORMULA & MOLECULAR FORMULA
An empirical formula represents the simplest
whole-number ratio of various atoms present in
a compound, whereas, the molecular formula
shows the exact number of different types of
atoms present in a molecule of a compound.
Example:
Empirical formula 👉 `CH_2O`
Molecular formula 👉 `C_6H_12O_6`
👉 If the mass per cent of various elements
present in a compound is known, its empirical
formula can be determined. The molecular formula
can further be obtained if the molar mass is
known.
Q) A compound contains 4.07% hydrogen,
24.27% carbon, and 71.65% chlorine. Its
molar mass is 98.96 g. What are its
empirical and molecular formulas?
Solution:
👉 Conversion of mass per cent
to grams
Let 100 g of the compound
then, 4.07g hydrogen, 24.27g carbon and
71.65g chlorine are present
👉 Convert into the number of moles of
each element.
Divide the masses by the respective atomic masses of various
elements.
Moles of hydrogen `= frac{4.07 g}{1.008g} = 4.04`
Moles of carbon `= frac{24.27 g}{ 12. 01g} = 2. 021`
Moles of chlorine `= frac{71.65g}{35. 453g} =2. 021`
👉 Divide each of the mole values
obtained above by the smallest number
amongst them
Since `2.021` is smallest value, division by
it gives a ratio of `2:1:1` for `H:C:Cl `.
`CH_2Cl` is, thus, the empirical formula of
the above compound.
👉 Writing empirical formula
For `CH_2Cl`, empirical formula mass is
`12.01 + (2 × 1.008) + 35.453
= 49.48 g`
Divide Molar mass by empirical
formula mass
Molar mass/ Empirical formula mass `= frac{98.96g}{49.48g} = 2 = n`
Multiply empirical formula by `n` obtained above to get the molecular
formula
Empirical formula `= CH_2Cl, n = 2`. Hence
molecular formula is `C_2H_4Cl_2`.
Reactions in Solutions
The concentration of a solution
or the amount of substance present in its
given volume can be expressed in any of the
following ways.
1. Mass per cent or weight per cent (w/w %)
2. Mole fraction
3. Molarity
4. Molality
1. Mass per cent
It is obtained by using the following relation:
2. Mole Fraction
It is the ratio of number of moles of a particular
component to the total number of moles of the
solution. If a substance ‘A’ dissolves in
substance ‘B’ and their number of moles are `n_A` and `n_B`, respectively, then the mole fractions
of `A` and `B` are given as:
3. Molarity
It is the most widely used unit and is denoted
by M. It is defined as the number of moles of
the solute in 1 litre of the solution. Thus,
4. Molality
It is defined as the number of moles of solute
present in 1 kg of solvent. It is denoted by m.
References










Wow
ReplyDeleteGood work 💯🤟💯👌
ReplyDeleteNice notes to understand this chapter 👍👍
ReplyDeleteNice
ReplyDeleteIt is very useful and better Gurukul and akash byjus
ReplyDelete