ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES AND TECHNIQUES
CLASSIFICATION OF ORGANIC
COMPOUNDS
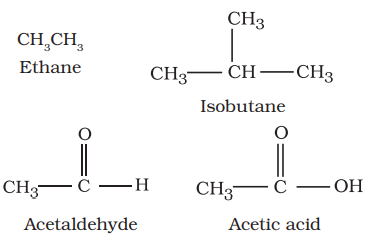
I. Acyclic or open-chain compounds
These compounds are also called as aliphatic
compounds and consist of straight or
branched chain compounds, for example:
II Cyclic or closed chain or ring
compounds
(a) Alicyclic compounds
Alicyclic (aliphatic cyclic) compounds contain
carbon atoms joined in the form of a ring (homocyclic).
Sometimes atoms other than carbon
are also present in the ring (heterocyclic).
Tetrahydrofuran given below is an example of
this type of compound:
(b) Aromatic compounds
Aromatic compounds are special types of
compounds. These include
benzene and other related ring compounds
(benzenoid).
Benzene has a homocyclic hexagonal ring of six carbon atoms with three double bonds in the alternate positions.
Like alicyclic compounds,
aromatic comounds may also have hetero
atom in the ring. Such compounds are called
heterocyclic aromatic compounds. Some of the
examples of various types of aromatic
compounds are:
Benzenoid aromatic compounds
Non-benzenoid compound
Heterocyclic aromatic compounds
Functional Group
The functional group is an atom or a group of
atoms joined to the carbon chain which is
responsible for the characteristic chemical
properties of organic compounds.
Examples are:
hydroxyl group (–OH), aldehyde
group (–CHO) and carboxylic acid group
(–COOH), etc.
Homologous Series
A group or a series of organic compounds each
containing a characteristic functional group
forms a homologous series
👉 Members
of the series are called homologues.
👉The successive members differ from each other
in molecular formula by a `–CH_2` unit.
Some of these are alkanes,
alkenes, alkynes, haloalkanes, alkanols,
alkanals, alkanones, alkanoic acids, amines etc.
Hydrocarbons
Compounds containing carbon
and hydrogen only are called hydrocarbons.
Saturated Hydrocarbon
A
hydrocarbon is termed saturated if it contains
only carbon-carbon single bonds.
The IUPAC
name for a homologous series of such
compounds is alkane.
Unsaturated Hydrocarbons
Unsaturated hydrocarbons are
those, which contain at least one carbon-carbon double or triple bond.
Trivial or common names
These
names are traditional and are considered as
trivial or common names. 👉 Organic compounds were assigned
names based on their origin or certain
properties.
Examples:
Citric acid is named so because it is found in citrus fruits.
Acid found in red ant is named formic acid
since the Latin word for ant is formica.
The IUPAC System of Nomenclature
IUPAC
(International Union of Pure and Applied
Chemistry)
In this
systematic nomenclature, the names are correlated
with the structure such that the reader or listener
can deduce the structure from the name.
👉 A systematic name of an organic compound is
generally derived by identifying the parent
hydrocarbon, alkyl groups, and the functional groups attached to it. See the example given below.
IUPAC Nomenclature of Alkanes
👉 Straight chain hydrocarbons
👉 Branched chain alkanes
👉 Cyclic Compounds
👉 Straight chain hydrocarbons
The names
of such compounds are based on their chain
structure, and end with suffix ‘-ane’ and carry
a prefix indicating the number of carbon
atoms present in the chain (except from `CH_4` to `C_4H_10`, where the prefixes are derived from
trivial names).
Examples:
Alkyl Group
An alkyl group is derived from
a saturated hydrocarbon by removing a
hydrogen atom from carbon.
Thus, `CH_4` becomes `-CH_3` and is called methyl group. An
alkyl group is named by substituting ‘yl’ for
‘ane’ in the corresponding alkane.
👉 Branched-chain hydrocarbons
In a
branched chain compound, small chains of
carbon atoms are attached at one or more
carbon atoms of the parent chain. The small
carbon chains (branches) are called alkyl
groups. For example:
The rules for naming them are given
below
Rule 1
The longest carbon chain in
the molecule is identified. In the example
(I) given below, the longest chain has nine
carbons and it is considered as the parent
or root chain. Selection of parent chain as
shown in (II) is not correct because it has only eight carbons.
Rule 2
The carbon atoms of the parent chain are
numbered to identify the parent alkane and
to locate the positions of the carbon atoms
at which branching takes place due to the
substitution of alkyl group in place of
hydrogen atoms. The numbering is done
in such a way that the branched carbon
atoms get the lowest possible numbers.
Thus, the numbering in the above example
should be from left to right (branching at
carbon atoms 2 and 6) and not from right
to left (giving numbers 4 and 8 to the
carbon atoms at which branches are
attached).
Rule 3
The names of alkyl groups attached
as a branch are then prefixed to the
name of the parent alkane and position
of the substituents is indicated by the
appropriate numbers. If different alkyl
groups are present, they are listed in
alphabetical order. Thus, name for the
compound shown above is: 6-ethyl-2-methylnonane. [Note: the numbers are separated from the groups by hyphens
and there is no break between methyl and nonane}]
Rule 4
If two or more identical substituent groups
are present then the numbers are
separated by commas. The names of
identical substituents are not repeated,
instead prefixes such as di (for 2), tri
(for 3), tetra (for 4), penta (for 5), hexa (for
6) etc. are used. While writing the name of
the substituents in alphabetical order,
these prefixes, however, are not considered.
Thus, the following compounds are
named as:
















Helpful Notes 👌
ReplyDeleteNice notes
ReplyDelete