The d- and f - Block Elements
Introduction
The d-block of the periodic table contains the elements
of groups 3-12 in which the d orbitals are
progressively filled in each of the four long periods.
The f-block consists of elements in which 4 f and 5 f
orbitals are progressively filled. They are placed in a
separate panel at the bottom of the periodic table. The
names transition metals and inner transition metals
are often used to refer to the elements of d-and
f-blocks respectively.
There are mainly four series of the transition metals
➤ 3d series (Sc to Zn)
➤ 4d series (Y to Cd)
➤ 5d series (La
and Hf to Hg)
➤ 6d series which has Ac and elements
from Rf to Cn.
The two series of the inner transition
metals; 4f (Ce to Lu) and 5f (Th to Lr) are known as
lanthanoids and actinoids respectively.
👉 Originally the name transition metals was derived
from the fact that their chemical properties were
transitional between those of s and p-block elements.
👉 According to IUPAC, transition metals are defined
as metals which have incomplete d subshell either in
neutral atom or in their ions.
👉 Zinc, cadmium and
mercury of group 12 have full `d^10` configuration in their
ground state as well as in their common oxidation states
and hence, are not regarded as transition metals.
Various precious metals such as silver, gold and
platinum and industrially important metals like iron,
copper and titanium belong to the transition metals series.
Position in the
Periodic Table
The d–block occupies the large middle section of the periodic table
flanked between s– and p– blocks in the periodic table. The d–orbitals
of the penultimate energy level of atoms receive electrons giving rise to
four rows of the transition metals, i.e., 3d, 4d, 5d and 6d.
Electronic
Configurations
of the d-Block
Elements Elements
👉 In general the electronic configuration of outer orbitals of these elements
is `(n-1)d^ {1–10}` `ns^{ 1–2}`. The `(n–1)` stands for the inner d orbitals which may
have one to ten electrons and the outermost ns orbital may have one
or two electrons.
This generalisation has several exceptions
because of very little energy difference between `(n-1)d` and `ns` orbitals. Furthermore, half and completely filled sets of orbitals are relatively
more stable. A consequence of this factor is reflected in the electronic
configurations of Cr and Cu in the 3d series.
For example, consider the
case of Cr, which has `3d^5 4s^1` configuration instead of `3d^4
4s^2` ; the
energy gap between the two sets (3d and 4s) of orbitals is small enough
to prevent electron entering the 3d orbitals. Similarly in case of Cu, the
configuration is `3d^10 4s^1` and not `3d ^9
4s^2` .
👉 The electronic configurations of outer orbitals of Zn, Cd, Hg and Cn
are represented by the general formula `(n-1)d^10
ns^2` . The orbitals in
these elements are completely filled in the ground state as well as in
their common oxidation states. Therefore, they are not regarded as
transition elements.
👉 With partly
filled d orbitals these elements exhibit certain characteristic properties
such as display of a variety of oxidation states, formation of coloured
ions and entering into complex formation with a variety of ligands.
👉 The transition metals and their compounds also exhibit catalytic
property and paramagnetic behaviour.
👉 There are greater similarities in the properties of the transition
elements of a horizontal row in contrast to the non-transition elements.
However, some group similarities also exist.
Melting and Boiling Points
Transition metals have very high melting and boiling points. It is clear from the figure that the melting points of these metals rise to a maximum value and then decrease with an increase in atomic number except for
anomalous values of Mn and Tc.
The high melting and boiling points of these metals are due to strong metallic bonds between the atoms of these elements. This is also evident from the fact that these metals have high enthalpies of atomization. The metallic bond is formed due to the interaction of electrons in the outermost orbitals. The strength of bonding is roughly related to the number of unpaired electrons. In general, the greater the number of valence electrons, the stronger the metallic bonding, and consequently, melting points are high.
Therefore, as we move along a particular series, the metallic strength increases upto the middle with increasing availability of unpaired electrons upto `d^5` configuration (e.g. Sc has 1, Ti has 2, V has 3, Cr has 5 unpaired electrons) and then decreases with decreasing availability of unpaired d-electrons (e.g. Fe has 4, Co has 3 unpaired electrons and so on). Therefore, the melting points decrease after the middle because of increase of pairing of electrons. The elements of group 12 (zinc, cadmium and mercury) are quite soft with low melting points. Mercury is a liquid at room temperature and melts at -38°C. These three elements behave typically because there are no unpaired electrons available for metallic bonding and, therefore, their melting points are low.
Why do the transition elements exhibit higher enthalpies of
atomisation?
Because of large number of unpaired electrons in their atoms they
have stronger interatomic interaction and hence stronger bonding
between atoms resulting in higher enthalpies of atomisation.
Variation in Atomic and Ionic Sizes of Transition Metals
An interesting point emerges when atomic sizes of one series are compared with those of the corresponding elements in the other series. The curves in Fig. show an increase from the first `(3d)` to the second `(4d)` series of the elements but the radii of the third `(5d)` series are virtually the same as those of the corresponding members of the second series.
This phenomenon is associated with the intervention of the `4f` orbitals which must be filled before the `5d` series of elements begin. The filling of `4f` before `5d` orbital results in a regular decrease in atomic radii called Lanthanoid contraction which essentially compensates for the expected increase in atomic size with increasing atomic number. The net result of the lanthanoid contraction is that the second and the third `d` series exhibit similar radii (e.g., Zr 160 pm, Hf 159 pm).
However, the shielding of one `4f` electron by another is less than that of one d electron by another, and as the nuclear charge increases along the series, there is fairly regular decrease in the size of the entire `4f^n` orbitals.
Density
👉 The decrease in metallic radius coupled with increase in atomic mass results in a general increase in the density of these elements. Thus, from titanium (Z = 22) to copper (Z = 29) the significant increase in the density.
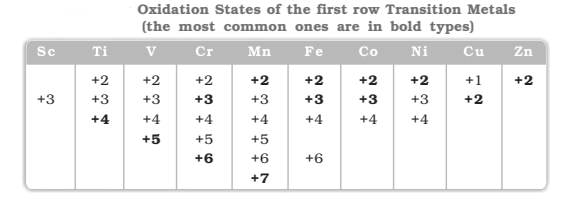
Oxidation States
One of the notable features of a transition elements is the great variety of oxidation states.
👉 The elements which give the greatest number of oxidation states
occur in or near the middle of the series. Manganese, for example,
exhibits all the oxidation states from `+2` to `+7`.
👉 The lesser number of
oxidation states at the extreme ends stems from either too few electrons
to lose or share (Sc, Ti) or too many d electrons (hence fewer orbitals
available in which to share electrons with others) for higher valence
(Cu, Zn).
👉 Early in the series scandium(II) is virtually unknown
and titanium (IV) is more stable than Ti(III) or Ti(II). At the other end,
the only oxidation state of zinc is `+2` (no d electrons are involved).
👉 The maximum oxidation states of reasonable stability correspond in value to the sum of the s and d electrons upto manganese `(Ti^{iv}, V^v, Cr^{vi}, Mn^{vii})` followed by a rather abrupt decrease in stability of
higher oxidation states, so that the typical species to follow are `(Fe^{ii,iii}, Co^{ii,iii}, Ni^{ii}, Cu^{i,ii}, Zn^{ii})`.
👉 The variability of oxidation states, a characteristic of transition
elements, arises out of incomplete filling of d orbitals in such a way
that their oxidation states differ from each other by unity, e.g., `V^{ii}, V^{iii}, V^{iv}, V^{v}`. This is in contrast with the variability of oxidation states of non
transition elements where oxidation states normally differ by a unit
of two.
Name a transition element which does not exhibit variable
oxidation states.
Scandium (Z = 21) does not exhibit variable oxidation states.
Formation of Coloured Ions
👉 When an electron from a lower energy d orbital is excited to a higher energy d orbital, the energy of excitation corresponds to the frequency of light absorbed . This frequency generally lies in the visible region. The colour observed corresponds to the complementary colour of the light absorbed.
👉 The frequency of the light absorbed is determined by the nature of the ligand.
👉 In aqueous solutions where water molecules are the ligands, the colours of the ions observed are listed in Table
Magnetic Properties
When a magnetic field is applied to substances, mainly two types of magnetic behaviour are observed: diamagnetism and paramagnetism
👉Diamagnetic substances are repelled by the applied field while the paramagnetic substances are attracted.
👉 Substances which are attracted very strongly are said to be ferromagnetic. In fact,
ferromagnetism is an extreme form of paramagnetism.
👉 Paramagnetism arises from the presence of unpaired electrons, each
such electron having a magnetic moment associated with its spin angular
momentum and orbital angular momentum.
For the compounds of the
first series of transition metals, the contribution of the orbital angular
momentum is effectively quenched and hence is of no significance. For
these, the magnetic moment is determined by the number of unpaired
electrons and is calculated by using the ‘spin-only’ formula, i.e.,
`\mu = sqrt{ n(n + 2)}`
where `n` is the number of unpaired electrons and `µ` is the magnetic
moment in units of Bohr magneton (BM). A single unpaired electron
has a magnetic moment of 1.73 Bohr magnetons (BM).
The magnetic moment increases with the increasing number of
unpaired electrons. Thus, the observed magnetic moment gives a useful
indication about the number of unpaired electrons present in the atom,
molecule or ion. The magnetic moments calculated from the ‘spin-only’
formula and those derived experimentally for some ions of the first row
transition elements are given in Table
Formation
of Complex
Compounds
👉Complex compounds are those in which the metal ions bind a number
of anions or neutral molecules giving complex species with
characteristic properties.
A few examples are: `[Fe (CN)_6 ]^{3-}`, `[Fe (CN)_6 ]^{4-}`, `[Cu(NH_3)_4]^{2+}`, `[PtCl_4]^{2-}`
👉 The transition metals form a large
number of complex compounds. This is due to their high ionic charges and the
availability of d orbitals for bond formation
Catalytic
Properties
👉👉 A catalyst is a substance that speeds up a chemical reaction without being consumed in the process. It lowers the activation energy required for the reaction to occur, making it happen more quickly.
👉 The transition metals and their compounds are known for their catalytic
activity. This activity is ascribed to their ability to adopt multiple
oxidation states and to form complexes.
Examples:
1. Vanadium(V) oxide (in Contact
Process) 👉 formation of sulphuric acid ,
2. Finely divided iron (in Haber’s Process) 👉 ammonia
3. Nickel (in Catalytic
Hydrogenation) 👉 unsaturated fat to saturated fat.
👉 Catalysts at a solid surface
involve the formation of bonds between reactant molecules and atoms
of the surface of the catalyst (first row transition metals utilise 3d and
4s electrons for bonding). This has the effect of increasing the
concentration of the reactants at the catalyst surface and also weakening
of the bonds in the reacting molecules (the activation energy is lowering).
👉 The transition metal ions can change their oxidation states,
they become more effective as catalysts.
Examples
`2I^{-} + S_2O_8^{2-} \rightarrow I_2 + 2SO_4^{2-}`
For example, iron(III) catalyses
the reaction between iodide and persulphate ions
`2Fe^{3+} + 2I^{-} \rightarrow 2Fe^{2+} + I_2`
`2Fe^{2+} + S_2O_8^{2-} \rightarrow 2Fe^{3+} + 2SO_4^{2-}`
Formation of Interstitial Compounds
👉👉Interstitial compounds are those which are formed when small atoms like H, C or N are trapped inside the crystal lattices of metals.
👉 They are usually non-stoichiometric and are neither typically ionic nor covalent, for example, `TiC, Mn_4N, Fe_3H, VH_0.56, TiH_1.7`, etc.
👉 The formulas quoted do not, of course, correspond to any normal oxidation state of the metal.
Because of the nature of their composition, these compounds
are referred to as interstitial compounds. The principal physical and
chemical characteristics of these compounds are as follows:
(i) They have high melting points, higher than those of pure metals.
(ii) They are very hard, some borides approach diamonds in hardness.
(iii) They retain metallic conductivity.
(iv) They are chemically inert.
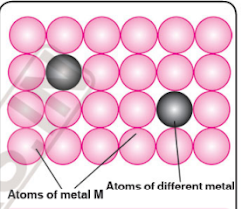
Alloy Formation
👉👉 An alloy is a blend of different metals, or a metal mixed with other elements, resulting in a new material with improved properties.
Alloys may be homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of the other.
👉 Alloys are formed by atoms with metallic radii that are within about 15 per cent of each other.
👉 Because of similar radii and other characteristics
of transition metals the atoms of one metal can substitute the atoms of other atoms.
👉 The alloys so formed are hard and have often high melting points
Examples:
The best
known are ferrous alloys: chromium, vanadium, tungsten, molybdenum
and manganese are used for the production of a variety of steels and
stainless steel. Alloys of transition metals with non-transition metals
such as brass (copper-zinc) and bronze (copper-tin), are also of
considerable industrial importance
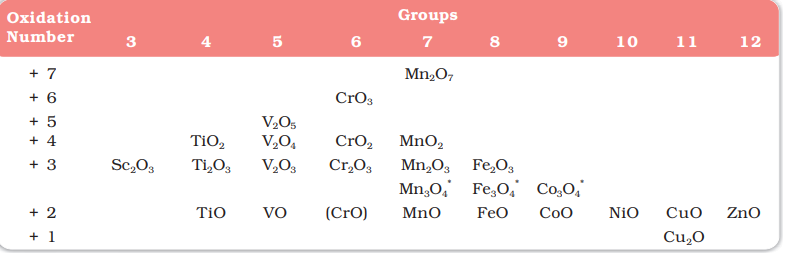
Oxides of Metals
👉These oxides are generally formed by the reaction of metals with oxygen at high temperatures.
👉 All the metals except scandium form `MO` oxides which are ionic.
👉 The highest oxidation number in the
oxides, coincides with the group number and is attained in `Sc_2O_3` to `Mn_2O_7` .
👉 Beyond group 7, no higher oxides of iron above `Fe_2O_3` are
known.
👉 Besides the oxides `(V_2O_5, V_2O_4, TiO_2)`, the oxocations stabilise `V^v` as `VO_2^+`, `V^{iv}` as `VO^{2+}`, `Ti^{iv}` as `TiO^{2+}`.
👉👉 Oxoanions are negatively charged ions composed of oxygen and other elements, forming essential compounds in chemistry with distinct structures and properties.
👉👉 Oxocations are positively charged ions (cations) formed by combining oxygen with other elements, influencing chemical reactivity and bonding in compounds.
👉 As the oxidation number of a metal increases, its ionic character
decreases. In the case of `Mn, Mn_2O_7` is a covalent green oil. . Even `CrO_3` and `V_2O_`5` have low melting points.
O.N ↑ I.C ↓ Cov.C ↑
O.N ↑ M.P ↓
👉 In these higher oxides, the acidic
character is predominant. O.N ↑ Ac ↑
Thus, `Mn_2O_7` gives `HMnO_4` and `CrO_3` gives `H_2CrO_4` and `H_2Cr_2O_7`.
`Mn_2O_7 + H_2O \rightarrow 2HMnO_4`
`CrO_3 + H_2O \rightarrow H_2CrO_4`
👉 `V_2O_5` is, however, amphoteric though mainly acidic and it gives `VO_4^{3-}` as well as `VO_2^{+}` salts.
👉 In vanadium there is gradual change from the basic `V_2O_3` to less basic `V_2O_4` and to amphoteric `V_2O_5`, `V_2O_4` dissolves in acids
to give `VO^{2+}` salts.
👉 `V_2O_5` reacts with alkalies as well as acids
to give `VO_4^{3-}` and `VO_4^{+}` respectively.
👉 The well characterised `CrO` is basic
but `Cr_2O_3` is amphoteric.
Potassium dichromate `K_2Cr_2O_7`
Potassium dichromate is a very important chemical used in leather
industry and as an oxidant for preparation of many azo compounds.
👉 Dichromates are generally prepared from chromate, which in turn are
obtained by the fusion of chromite ore (`FeCr_2O_4` ) with sodium or
potassium carbonate in free access of air. The reaction with sodium
carbonate occurs as follows:
`4 FeCr_2O_4 + 8 Na_2CO_3 + 7 O_2 → 8 Na_2CrO_4 + 2 Fe_2O_3 + 8 CO_2`
👉 The yellow solution of sodium chromate is filtered and acidified
with sulphuric acid to give a solution from which orange sodium
dichromate, `Na_2Cr_2O_7
. 2H_2O` can be crystallised.
`2Na_2CrO_4
+ 2 H^+ → Na_2Cr_2O_7
+ 2 Na^+
+ H_2O`
👉 Sodium dichromate is more soluble than potassium dichromate.
The latter is therefore, prepared by treating the solution of sodium
dichromate with potassium chloride
`Na_2Cr_2O_7
+ 2 KCl → K_2Cr_2O_7
+ 2 NaCl`
Orange crystals of potassium dichromate crystallise out.
👉 The chromates and dichromates are interconvertible in aqueous solution depending upon pH of the solution. The oxidation state of chromium in chromate and dichromate is the same.
`2 CrO_4^{2–} + 2H^+ → Cr_2O_7^{2–} + H_2O` (pH↓)
`Cr_2O_7^{ 2–} + 2 OH^{ -} → 2 CrO_4 ^{2–} + H_2O` (pH ↑)
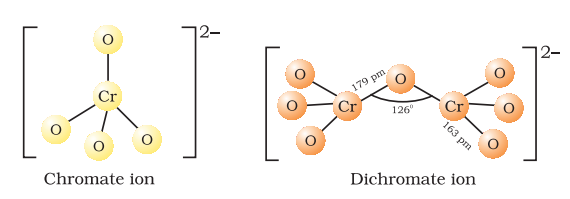
The structures of chromate ion, `CrO_4^{2–}` and the dichromate ion, `Cr_2O_7^{2–}`
👉The chromate ion is tetrahedral whereas the dichromate ion consists of two tetrahedra sharing one corner with Cr–O–Cr bond angle of `126°`.
👉 Sodium and potassium dichromates are strong oxidising agents; the sodium salt has a greater solubility in water and is extensively used as an oxidising agent in organic chemistry. Potassium dichromate is used as a primary standard in volumetric analysis. In acidic solution, its oxidising action can be represented as follows:
`Cr_2O_7^{2–} + 14H^+ + 6e^– → 2Cr_3^+ + 7H_2O` `(E^⊝ = 1.33V)`
👉 Acidified potassium dichromate will oxidise iodides to iodine, sulphides to sulphur, tin(II) to tin(IV) and iron(II) salts to iron(III). The half-reactions are noted below:
👉 The full ionic equation may be obtained by adding the half-reaction for potassium dichromate to the half-reaction for the reducing agent, for e.g.,
`Cr_2O_7^{ 2–} + 14 H^+ + 6 Fe^{2+} → 2 Cr^{3+} + 6 Fe^{3+} + 7 H_2O`
Potassium permanganate` KMnO_4`
👉 Potassium permanganate is prepared by fusion of `MnO_2` with an alkali metal hydroxide and an oxidising agent like `KNO_3`. This produces the dark green `K_2MnO_4` which disproportionates in a neutral or acidic solution to give permanganate.
`2MnO_2 + 4KOH + O_2 → 2K_2MnO_4 + 2H_2O`
`3MnO_4^{ 2–} + 4H^+ → 2MnO_4^– + MnO_2 + 2H_2O`
👉 Commercially it is prepared by the alkaline oxidative fusion of `MnO_2` followed by the electrolytic oxidation of manganate (Vl)
👉 In the laboratory, a manganese (II) ion salt is oxidised by
peroxodisulphate to permanganate.
`2Mn^{2+} + 5S_2O_8^{ 2–} + 8H_2O → 2MnO_4^ –
+ 10SO_4^ {2–} + 16H^+`
👉 Potassium permanganate forms dark purple (almost black) crystals which
are isostructural with those of `KClO_4` . The salt is not very soluble in water
(6.4 g/100 g of water at 293 K), but when heated it decomposes at 513 K.
`2KMnO_4 → K_2MnO_4
+ MnO_2
+ O_2`
👉 It has two physical properties of considerable interest: its intense colour
and its diamagnetism along with temperature-dependent weak
paramagnetism.
👉 The manganate and permanganate ions are tetrahedral; the `π-` bonding takes place by overlap of `p` orbitals of oxygen with `d` orbitals
of manganese. The green manganate is paramagnetic because of one
unpaired electron but the permanganate is diamagnetic due to the
absence of unpaired electron.
👉 Acidified permanganate solution oxidises oxalates to carbon dioxide,
iron(II) to iron(III), nitrites to nitrates and iodides to free iodine.
The half-reactions of reductants are:
👉 If we represent the reduction of permanganate to manganate,
manganese dioxide and manganese(II) salt by half-reactions,
We can very well see that the hydrogen ion concentration of the
solution plays an important part in influencing the reaction.
👉 A few important oxidising reactions of `KMnO_4` are given below:
1. In acid solutions:
2. In neutral or faintly alkaline solutions:
THE INNER TRANSITION ELEMENTS ( f-BLOCK)
👉The f-block consists of the two series, lanthanoids (the fourteen elements
following lanthanum) and actinoids (the fourteen elements following
actinium).
👉 Because lanthanum closely resembles the lanthanoids, it is
usually included in any discussion of the lanthanoids for which the
general symbol Ln is often used. Similarly, a discussion of the actinoids
includes actinium besides the fourteen elements constituting the series.
👉 The lanthanoids resemble one another more closely than do the members
of ordinary transition elements in any series. They have only one stable
oxidation state and their chemistry provides an excellent opportunity to
examine the effect of small changes in size and nuclear charge along a
series of otherwise similar elements.
👉 The chemistry of the actinoids is, on
the other hand, much more complicated. The complication arises partly
owing to the occurrence of a wide range of oxidation states in these
elements and partly because their radioactivity creates special problems
in their study.
👉 The
Lanthanoids
The names, symbols, electronic configurations of atomic and some
ionic states and atomic and ionic radii of lanthanum and lanthanoids
(for which the general symbol Ln is used) are given in Table.
👉 Electronic
Configurations `(n-2)f^{1-14} (n-1)d^{0-1} ns^2`
It may be noted that atoms of these elements have electronic
configuration with `6s^2` common but with variable occupancy of `4f` level. However, the electronic configurations of all the tripositive
ions (the most stable oxidation state of all the lanthanoids) are of the
form `4f^n` (n = 1 to 14 with increasing atomic number).
👉 Atomic and
Ionic Sizes
The overall decrease in atomic and ionic radii from lanthanum to
lutetium (the lanthanoid contraction) is a unique feature in the
chemistry of the lanthanoids. It has far reaching
consequences in the chemistry of the third
transition series of the elements.
The decrease
in atomic radii (derived from the structures of
metals) is not quite regular as it is regular in `M ^{3+}` ions (Fig.).
This contraction is, of
course, similar to that observed in an ordinary
transition series and is attributed to the same
cause, the imperfect shielding of one electron
by another in the same sub-shell.
Oxidation
States
👉All lanthanoids exhibit a common stable oxidation state of `+3`. However, occasionally `+2` and `+4` ions in solution or in solid
compounds are also obtained. This irregularity (as in ionisation
enthalpies) arises mainly from the extra stability of empty, half-filled
or filled f subshell.
👉The formation of `Ce^{IV}` is favoured by its
noble gas configuration, but it is a strong oxidant reverting to the
common `+3` state.
The `E^o` value for `Ce^{4+}`/ `Ce^{3+}` is `+ 1.74 V` which
suggests that it can oxidise water.
`Ce^{4+} + e^{-} \rightarrow Ce^{3+}`
However, the reaction rate is very
slow and hence `Ce(IV)` is a good analytical reagent.
👉 Pr, Nd, Tb and Dy
also exhibit `+4` state but only in oxides, `MO_2` .
👉 `Eu^{2+}` is formed by losing
the two `s` electrons and its `f^7` configuration accounts for the formation
of this ion. However, `Eu^{2+}` is a strong reducing agent changing to the
common `+3` state.
`Eu^{2+} \rightarrow Eu^{3+} + e^{-}`
👉 Similarly `Yb^{2+}` which has `f^{14}` configuration is a
reductant.
`Yb^{2+} \rightarrow Yb^{3+} + e^{-}`
👉`Tb^{IV}` has half-filled `f`-orbitals and is an oxidant.
`Tb^{4+} + e^{-} \rightarrow Tb^{3+}`
👉The
behaviour of samarium is very much like europium, exhibiting both `+2` and `+3` oxidation states.
⇒ Oxidising agents `Ce^{4+}, Tb^{4+}`
`Ce^{4+} + e^{-} \rightarrow Ce^{3+}`
`Tb^{4+} + e^{-} \rightarrow Tb^{3+}`
⇒ Reducing agents `Eu^{2+}, Yb^{2+}`
`Eu^{2+} \rightarrow Eu^{3+} + e^{-}`
`Yb^{2+} \rightarrow Yb^{3+} + e^{-}`
General
Characteristics
👉All the lanthanoids are silvery white soft metals and tarnish rapidly in air.
The hardness increases with increasing atomic number, samarium being
steel hard. Their melting points range between 1000 to 1200 K but
samarium melts at 1623 K. They have typical metallic structure and are
good conductors of heat and electricity. Density and other properties
change smoothly except for Eu and Yb and occasionally for Sm and Tm.
👉Many trivalent lanthanoid ions are coloured both in the solid state
and in aqueous solutions. Colour of these ions may be attributed to
the presence of `f` electrons. Neither `La^{3+}` nor `Lu^{3+}` ion shows any colour
but the rest do so. However, absorption bands are narrow, probably
because of the excitation within `f` level.
👉The lanthanoid ions other
than the `f^0` type `(La^{3+} & Ce^{4+})` and the `f^14` type `(Yb^{2+} & Lu^{3+})` are
all paramagnetic.
👉The first ionisation enthalpies of the lanthanoids are around `600 kJ mol^{–1}`, the second about `1200 kJ mol^{–1}` comparable with those
of calcium.
The variation of third ionisation enthalpies show some stabilities of empty `(f^0)`, half-filled `(f^7)` and completely filled `(f^14)`orbitals `f ` level. This is indicated from the abnormally low value of the third
ionisation enthalpy of lanthanum `(4f^0)`, gadolinium `(4f^7)` and lutetium `(4f^14)`.
👉In their chemical behaviour, in general, the earlier members of the series
are quite reactive similar to calcium but, with increasing atomic number,
they behave more like aluminium. Values for E
V
for the half-reaction:
`Ln^{3+}(aq) + 3e^– → Ln(s)`
are in the range of –2.2 to –2.4 V
except for Eu for which the value is
– 2.0 V. This is, of course, a small
variation.
Chemical Reactions
👉The metals combine with
hydrogen when gently heated in the
gas.
👉The carbides, `Ln_3C, Ln_2C_3` and `LnC_2` are formed when the metals are heated
with carbon.
👉 They liberate hydrogen
from dilute acids and burn in halogens
to form halides.
👉They form oxides `M_2O_3` and hydroxides `M(OH)_3` . The
hydroxides are definite compounds, not
just hydrated oxides. They are basic
like alkaline earth metal oxides and
hydroxides.
Their general reactions are
depicted in Fig.
The Actinoids
👉The actinoids include the fourteen elements from Th to Lr.
👉The actinoids are radioactive elements and the earlier members have
relatively long half-lives, the latter ones have half-life values ranging from
a day to 3 minutes for lawrencium (Z =103). The latter members could be
prepared only in nanogram quantities. These facts render their study
more difficult.
Electronic
Configurations
👉All the actinoids are believed to have the electronic configuration of `7s^2` and variable occupancy of the `5f` and `6d` subshells.
👉The fourteen electrons
are formally added to `5f`, though not in thorium (Z = 90) but from Pa
onwards the `5f` orbitals are complete at element 103.
👉The irregularities in
the electronic configurations of the actinoids, like those in the lanthanoids
are related to the stabilities of the `f ^0 , f ^7` and `f^14` occupancies of the `5f` orbitals. Thus, the configurations of Am and Cm are `[Rn] 5f ^7
7s^ 2` and `[Rn] 5f ^{7} 6d^1
7s^ 2`.
👉Although the `5f` orbitals resemble the `4f` orbitals in their
angular part of the wave-function, they are not as buried as `4f` orbitals
and hence `5f `electrons can participate in bonding to a far greater extent.
Ionic Sizes
👉The general trend in lanthanoids is observable in the actinoids as well.
There is a gradual decrease in the size of atoms or `M^{3+}` ions across the
series. This may be referred to as the actinoid contraction (like lanthanoid
contraction). The contraction is, however, greater from element to element
in this series resulting from poor shielding by 5f electrons.
Oxidation
States
There is a greater range of oxidation states, which is in part attributed to
the fact that the `5f, 6d` and `7s` levels are of comparable energies.
👉The actinoids show in general `+3` oxidation state. The elements, in the
first half of the series frequently exhibit higher oxidation states. For example,
the maximum oxidation state increases from `+4` in Th to `+5, +6` and `+7` respectively in Pa, U and Np but decreases in succeeding elements.
👉The actinoids resemble the lanthanoids in having more compounds
in `+3` state than in the `+4` state. However, `+3` and `+4` ions tend to hydrolyse.
👉Because the distribution of oxidation states among the actinoids is so
uneven and so different for the former and later elements, it is unsatisfactory
to review their chemistry in terms of oxidation states.
General
Characteristics
and Comparison
with Lanthanoids
👉The actinoid metals are all silvery in appearance but display
a variety of structures. The structural variability is obtained
due to irregularities in metallic radii which are far greater
than in lanthanoids.
👉The actinoids are highly reactive metals, especially when finely divided.
👉The action of boiling water on them, for example, gives a mixture of oxide
and hydride and combination with most non metals takes place at
moderate temperatures.
👉Hydrochloric acid attacks all metals but most are
slightly affected by nitric acid owing to the formation of protective oxide
layers; alkalies have no action.
👉The magnetic properties of the actinoids are more complex than those
of the lanthanoids. Although the variation in the magnetic susceptibility
of the actinoids with the number of unpaired 5 f electrons is roughly
parallel to the corresponding results for the lanthanoids, the latter have
higher values.
👉It is evident from the behaviour of the actinoids that the ionisation
enthalpies of the early actinoids, though not accurately known, but are
lower than for the early lanthanoids. This is quite reasonable since it is to
be expected that when 5f orbitals are beginning to be occupied, they will
penetrate less into the inner core of electrons The 5f electrons, will therefore,
be more effectively shielded from the nuclear charge than the 4f electrons
of the corresponding lanthanoids. Because the outer electrons are less
firmly held, they are available for bonding in the actinoids.
👉A comparison of the actinoids with the lanthanoids, with respect to
different characteristics as discussed above, reveals that behaviour similar
to that of the lanthanoids is not evident until the second half of the
actinoid series. However, even the early actinoids resemble the lanthanoids
in showing close similarities with each other and in gradual variation in
properties which do not entail change in oxidation state. The lanthanoid
and actinoid contractions, have extended effects on the sizes, and
therefore, the properties of the elements succeeding them in their
respective periods. The lanthanoid contraction is more important because
the chemistry of elements succeeding the actinoids are much less known
at the present time.















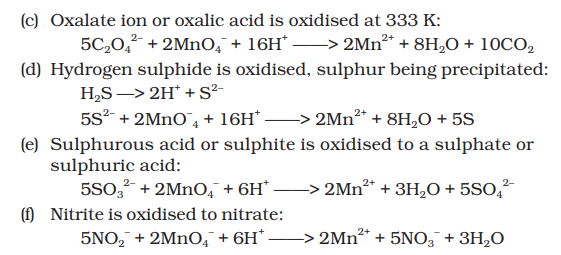







Nice notes
ReplyDeleteThank you dear
ReplyDeleteVery easy or thought full notes
ReplyDeleteIt get me ranked marks
Thank you Saurav
DeleteGood notes👍
ReplyDeleteThank you Piyush
DeleteMind blowing notes 👏👏👏👏
ReplyDeleteThank you Rajeev
DeleteVery nice notes
ReplyDelete